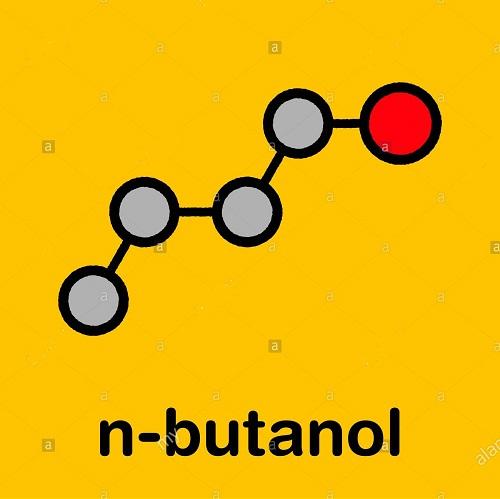
The polarity analysis of 1-Butanol
Description
1-Butanol is a vital chemical platform used as feedstock in the plastic industry, plasticizers, paints, binders, and food extractants. It can be used as an additive for gasoline, diesel, and kerosene or as an alternative fuel. Studies show that using 1-butanol for this purpose is much more advantageous than using ethanol[1]. 1-Butanol has higher energy density, lower vapor pressure than ethanol, improved miscibility with gasoline, lower water solubility, and less corrosive than ethanol. Various species of Clostridium bacteria, such as C. acetobutylicum, C. beijerinckii, C. saccharoperbutylacetonicum, and C. saccharobutylicum, are mainly used for 1-butanol production.
Polar analysis
1-butanol consists of all covalent bonds. Hence, it is a molecular compound. However, a polar protic -OH group and a polar C-O bond make it a molecular polar compound.
1-butanol or Butan-1-ol has the formula CH3-CH2-CH2-CH2-OH. While the OH part is polar, the non-polar C4H9 is attached to it. The greater the hydrocarbon portion, the more non-polar it becomes. Its lower homologs ( Methanol et al.) have lesser hydrocarbon portions and are more polar and water-soluble. Hence, we can consider 1-butanol to be non-polar due to its low water solubility.
1-octanol, 1-butanol, and Methanol
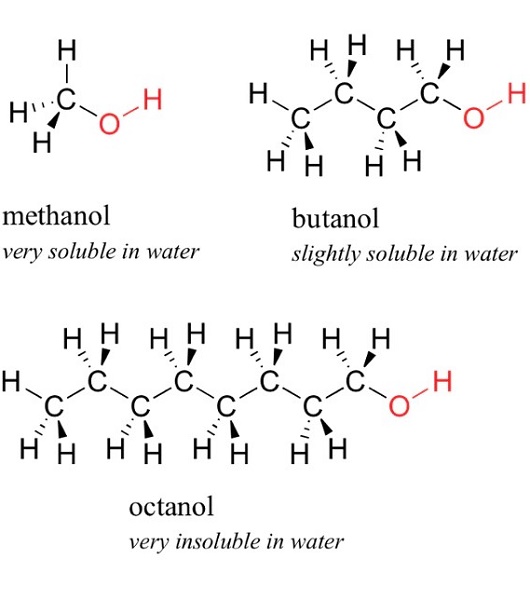
Since 1-octanol was the least polar among the 3 alcohols used in this part, it was found to be insoluble in water but soluble in hexane. This was due to the longer chain of carbons, making compounds more hydrocarbon-like. 1-Butanol with intermediate polarity was soluble in highly polar water and non-polar hexane, as 1-butanol can be either a polar or non-polar compound. 1-Butanol was polar based on the general rule of thumb that each polar group will allow up to 4 carbons to be soluble in water. Also, 1-butanol can be non-polar due to its carbon chains, which are attracted to the nonpolarity of the hexane. Methanol was the most polar among the 3 alcohols used in this part; hence, it was soluble in water as both water and methanol were polar.
References
[1] Dzida, M. “Thermophysical Properties of 1-Butanol at High Pressures.” Energies (2020).
You may like
Related articles And Qustion
See also
Lastest Price from 1-Butanol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 71-36-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00/kg2025-04-15
- CAS:
- 71-36-3
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons