What is 1-Butanol?
Overview
n-Butyl alcohol is a colourless flammable liquid with strong alcoholic odour. n-Butyl alcohol is a highly refractive liquid and burns with a strongly luminous flame. It is incompatible with strong acids, strong oxidising agents, aluminium, acid chlorides, acid anhydrides, copper, and copper alloys. n-Butyl alcohol has an extensive use in a large number of industries. For instance, it is used as solvent in industries associated with the manufacturing of paints, varnishes, synthetic resins, gums, pharmaceuticals, vegetable oils, dyes, and alkaloids. n-Butyl alcohol finds its use in the manufacture of artificial leather, rubber, plastic cements, shellac, raincoats, perfumes, and photographic films.
Chemical Properties
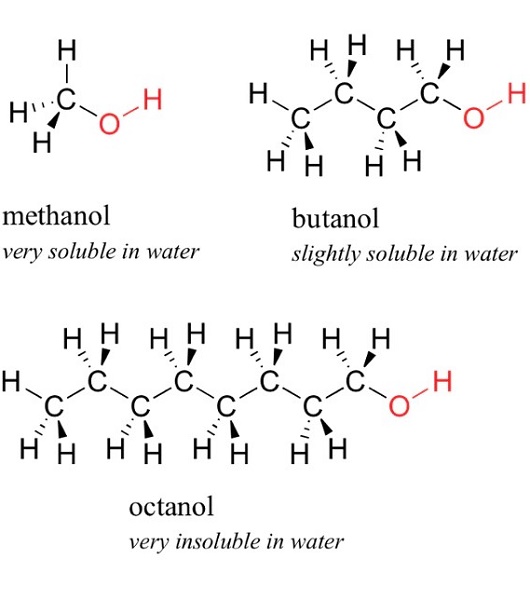
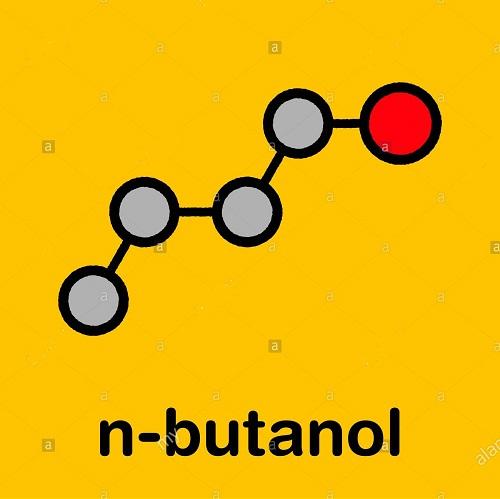
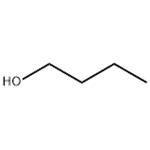
1-Butanol has the boiling point of being 117.7 ℃, the density (20 ℃) being 0.8109g/cm3, the freezing point being-89.0 ℃, flash point being 36~38 ℃, self-ignition point being 689F and the refractive index being (n20D) 1.3993. At 20 ℃, its solubility in water is 7.7% (by weight) while the water solubility in 1-butanol was 20.1% (by weight). It is miscible with ethanol, ether and other kinds of organic solvents. Two alcohols that are derived from butane: the primary alcohol butan-1-ol (CH3(CH2)2CH2OH) and the secondary alcohol butan-2-ol (CH3CH(OH)CH2CH3). Both are colorless volatile liquids used as solvents.
Discovery and Development
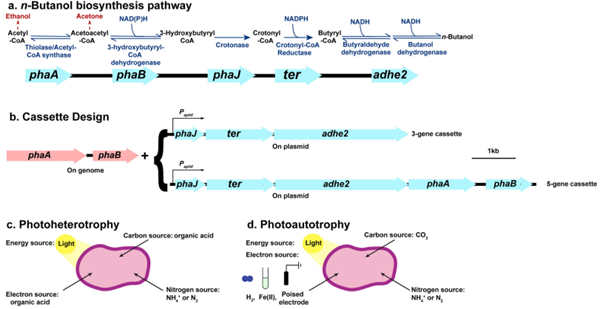
1-butanol was first discovered by C-A. Wurtz (French) from the fusel oil obtained from the fermentation process of alcohol in 1852. In 1913, the British Strange-Graham Companies have used corn as raw material for production of acetone through the fermentation process with butanol being the main byproduct. Later, due to the increasing demand for butanol, the fermentation production factory began to mainly synthesize n-butanol with acetone and ethanol being the major byproduct. During the Second World War, the German chemical company (Ruhr) began to apply propylene carboxyl method for the production of 1-butanol. With the rise of the oil industry in 1950s, the 1-butanol synthesis method had gotten rapid development with the propylene carboxyl method having the fastest speed.
Uses
1-Butanol is the most important in industries and the most extensively studied. 1-Butanol is a colorless liquid with a strong, mildly alcoholic odor. It is used in chemical derivatives and as a solvent for paints, waxes, brake fluid, and cleaners. 1-Butanol occurs naturally as a minor product of the fermentation of sugars and other carbohydrates and is present in many foods and beverages. It is also a permitted artificial flavorant in the United States, used in butter, cream, fruit, rum, whiskey, ice cream and ices, candy, baked goods, and cordials. It is also used in a wide range of consumer products.
1-Butanol is used as a solvent for many organic reactions in pharmaceutical and chemical industry, as a solvent in coating industries and also used as a diluent/reactant in the manufacture of urea-formaldehyde and melamine-formaldehyde resins. It is used in the production of drugs, alkaloids, additive in floor cleaners, consumer products and stain removers. It is used as a mobile phase in paper and thin layer chromatography. It is also used as an alternative for gasoline and diesel fuels.
1-Butanol is mainly used for the manufacture of the n-butyl plasticizers of phthalic acid, aliphatic dicarboxylic acid and phosphoric acid that are widely applied to various kinds of plastic and rubber products. It can also be used as the raw material of producing butyraldehyde, butyric acid, butyl-amine and butyl lactate in the field of organic synthesis. It can also be used as the extraction agent of oil, drugs (such as antibiotics, hormones and vitamins) and spices as well as the alkyd paint additives. It can be used as the solvent of organic dyes and printing ink and de-waxing agent.
tert-Butanol has been used for a variety of other purposes, including as the solvents of various paints and the raw material for producing the plasticizers, dibutyl phthalate. It can also be used for the manufacture of butyl acrylate, butyl acetate, and ethylene glycol butyl ether and also used as the extract of intermediates of organic synthesis and biochemical drugs and can also used in the manufacture of surfactants. Its steam can form explosive mixtures with air with the explosion limit being 3.7%~10.2% (volume fraction).
Health Hazard
Exposures to n-butyl alcohol by inhalation, ingestion, and/or skin absorption are harm ful. n-Butyl alcohol is an irritant, with a narcotic effect and a CNS depressant. Butyl alcohols have been reported to cause poisoning with symptoms that include, but are not limited to, irritation to the eyes, nose, throat, and the respiratory system. Prolonged exposure results in symptoms of headache, vertigo, drowsiness, corneal infl amma tion, blurred vision, photophobia, and cracked skin. It is advised that workers com ing in contact with n-butyl alcohol should use protective clothing and barrier creams. Occupational workers with pre-existing skin disorders or eye problems, or impaired liver, kidney or respiratory function may be more susceptible to the effects of the substance.
Related articles And Qustion
See also
Lastest Price from 1-Butanol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 71-36-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00/kg2025-04-15
- CAS:
- 71-36-3
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons