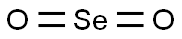Selenium Dioxide: Applications in Synthesis of Nanocrystals and Removal from Flue Gas
General Description
Selenium dioxide, a white crystalline solid with oxidizing properties, is utilized in various industrial applications. It serves as a crucial precursor in the synthesis of high-quality ZnSe nanocrystals, offering a green alternative to traditional methods. The compound is also effective in the removal of sulfur dioxide and trace selenium dioxide from flue gas, with calcium oxide demonstrating significant adsorption capabilities. Despite the potential toxicity of selenium dioxide, its unique properties make it a valuable component in technological advancements, showcasing versatility in tailored nanocrystal synthesis and environmental remediation processes.

Figure 1. Selenium dioxide
Overview
Selenium dioxide, chemically represented as SeO2, is a compound of selenium and oxygen. It exists as a white crystalline solid and exhibits oxidizing properties. Selenium dioxide finds various applications in industrial processes, including as a reagent in organic synthesis and as a catalyst in certain chemical reactions. Its unique properties make it a valuable component in several technological advancements. However, it should be handled with care due to its potential toxicity. 1
Applications in Synthesis of Nanocrystals
For the synthesis of the ZnSe nanocrystals, selenium dioxide(SeO2) was directly dissolved in ODE at 240 ◦C. This solution is stable for months and can be used effectively for the synthesis of nanocrystals. Cao et al. described a mechanism where Se0 was produced in the Se-containing reaction mixture upon reaction with long chain alkanes at high temperature.
First, ZnO reacted with OA to form a Zn–OA solution. Second, selenium dioxide powder was dissolved in ODE and then the Zn complex reacted with Se0 to form the ZnSe nanocrystals. However, the solubility of SeO2 in ODE is generally low and, for the synthesis of high quality nanocrystals, the nucleation and growth stages of the nanocrystals must be separated by the swift injection of precursors. The volume of the Se precursors can not be large, in order to facilitate the injection, and the amount of the Se precursor that participates in the reaction is limited by the small volume of the Se injection solution. In this report, the SeO2 was directly dissolved in ODE at an elevated temperature to act as the reaction solution. ZnO and OA reacted in ODE to form Zn–OA complex solution, which acted as the injection solution. This is different from the traditional injection method, where the chalcogen precursors are usually used as the injection solution and the solution with the Zn precursors usually acts as the reaction solution, so our method is termed as the “inverse injection method”. Using this approach, the Zn–OA complex has a high solubility in paraffin oil, so the amount of the Zn precursors injected into the reaction solution can be very large, which, together with the large amount of selenium dioxide dissolved in the large volume of the reaction solution, has led to the successful large scale synthesis of ZnSe nanocrystals. More than 2 g (contains the weight of surface ligands) of high quality ZnSe nanocrystals powder was synthesized with one reaction. The reaction yield of the nanocrystals was about 69%.2
Removal from Flue Gas
The removal of selenium dioxide from flue gas is a significant concern due to its harmful effects on the environment and human health. In the context of coal combustion, sulfur dioxide (SO2) and trace selenium dioxide are pollutants emitted into the atmosphere. This study focuses on the simultaneous removal of sulfur and trace selenium dioxide by calcium oxide (CaO) adsorption at medium temperatures, with particular emphasis on the mass transfer effects of sulfate product layers on trace elements. Experimental findings demonstrate that the presence of a product layer introduces additional mass transfer resistance to the sorbent-gas reaction process. However, as the concentration of SO2 decreases, the extent of CaO adsorption ability loss due to this factor diminishes. At trace gas concentrations, the loss of CaO adsorption ability becomes negligible. Furthermore, experiments investigating the adsorption of trace selenium dioxide gas by CaO indicate that the sulfate product layer, regardless of its thickness, does not significantly affect CaO's ability to adsorb trace selenium dioxide gas. Overall, these findings highlight the efficacy of calcium oxide in simultaneously removing sulfur dioxide and trace selenium dioxide from flue gas, with the understanding that the presence of a sulfate product layer may introduce some mass transfer resistance, but its impact diminishes at lower SO2 concentrations. Additionally, the presence of a sulfate product layer does not hinder CaO's ability to adsorb trace selenium dioxide gas. 3
Reference
1. Selenium dioxide. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 24007.
2. Shen H, Niu JZ, Wang H, Li X, Li LS, Chen X. Size- and shape-controlled synthesis of ZnSe nanocrystals using SeO2 as selenium precursor. Dalton Trans. 2010; 39(47): 11432-11438.
3. Li Y, Tong H, Zhuo Y, Chen C, Xu X. Simultaneous removal of SO2 and trace SeO2 from flue gas: effect of product layer on mass transfer. Environ Sci Technol. 2006;40(13):4306-4311.
You may like
Related articles And Qustion
Lastest Price from Selenium dioxide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7446-08-4
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 7446-08-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons