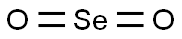Application and Pharmacology of Selenium dioxide
General description
Selenium dioxide appears as a white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3. Toxic by ingestion and inhalation. Inorganic compounds formed through the oxidation of selenium. White shiny acicular crystal, white or reddish shiny acicular crystal powder, with pungent smell. It tastes sour and has a burning sensation. The steam is yellowish green and spicy. Hygroscopic. Light and heat stability. Sublimation at 315 ℃. Easy to absorb dry hydrogen fluoride and hydrogen chloride.
Application and Pharmacology
It is used as oxidant, catalyst, chemical reagent of organic compounds and raw material for manufacturing various inorganic selenium compounds. Selenium Dioxide-Mediated Bromination of α,β-Unsaturated Ketones Using N‑Bromosuccinimide in the Presence of p‑Toluenesulfonic Acid: A Versatile Route for the Synthesis of α′-Bromo-4-arylbut-3-en-2-one and α′,α′-Dibromo-4-arylbut-3-en-2-one. An efficient method for the synthesis of α,β-unsaturated α′-bromoketones and α,β-unsaturated α′,α′-dibromoketones is described using N-bromosuccinimide (NBS) as the brominating agent mediated by selenium dioxide (SeO2) in the presence of p-toluenesulfonic acid (PTSA) monohydrate in toluene. The method is simple, employing easily available shelf reagents to afford a wide range of products in good yields. The method highlighted that simple fine-tuning of the reaction conditions and molar equivalents of the reactants easily affords either mono- or dibrominated products in excellent yields. A number of these products have not been reported in the literature. All of the reactions were carried out ingram-scale quantities[1].
Figure 1 Selenium Dioxide-Mediated Bromination of α,β-Unsaturated Ketones Using N‑Bromosuccinimide in the Presence of p‑Toluenesulfonic Acid
2. Study confirmed that selenium dioxide can significantly reduce the survivin protein in COC1 / DDP cells. Therefore, it is speculated that SeO2 may reverse the DDP resistance of ovarian cancer cell lines by down regulating the expression of survivin protein and controlling the programmed death of drug-resistant cells. In conclusion, selenium dioxide can significantly reverse the cisplatin resistance of human ovarian cancer cisplatin resistant cell line COC1 / DDP, induce tumor cell apoptosis, and down regulate the expression of survivin protein in tumor cells, which may be a new potential reversal agent of chemotherapy resistance. Recent studies have shown that apoptosis is related to acquired platinum resistance, and survivin gene is an apoptosis suppressor gene. Recent studies have found that its anti apoptotic effect increases the resistance of tumor cells to chemotherapy drugs. Selenium is an essential nutrient element in the organism. Foreign studies have found that selenium methionine can block the occurrence of cisplatin resistance near the first cisplatin chemotherapy, and selenium compounds can block the occurrence of cisplatin resistance at the glutathione level. In this study, different concentrations of selenium dioxide and certain concentrations of cisplatin were used to treat human ovarian cancer cisplatin resistant cell lines. Selenium compounds can block the occurrence of cisplatin resistance at glutathione level. The reversal effect of SeO2 on DDP resistance in ovarian cancer cell line. VRP is a classical positive control reversal agent commonly used in most drug resistance reversal studies at home and abroad[2].
Safety
After contact with skin, wash immediately with plenty of (to be specified by the manufacturer). In case of accident or if you feel unwell, seek medical advice immediately (show the lable where possible). This material and/or its container must be disposed of as hazardous waste. Avoid release to the environment. Refer to special instructions/Safety data sheets. Toxic by inhalation and if swallowed. Danger of cumulative effects. Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Selenium disulfide is highly toxic. No pharmacokinetic experiments have been done. After local application, it can be slightly absorbed through the skin. It is easy to be absorbed through the skin from the inflamed or damaged skin. Long term application on the damaged skin may cause systemic poisoning symptoms. Please be careful.
Reference
1.Lipon T. M., Marpna I. D. & Wanniang K. et al., "Selenium Dioxide-Mediated Bromination of α,β-Unsaturated Ketones UsingN-Bromosuccinimide in the Presence of p-Toluenesulfonic Acid: A Versatile Route for the Synthesis of α′-Bromo-4-arylbut-3-en-2-one and α′,α′-Dibromo-4-arylbut-3-en-2-one," ACS Omega, Vol.6, No.41(2021), pp.27466-27477.
2.Hu Jia, liusisun, Li Yun: Study on seo_2 reversing drug resistance of human ovarian cancer drug resistant cell lines, modern preventive medicine, 2010, issue 13, pp. 2569-2571.
You may like
Related articles And Qustion
See also
Lastest Price from Selenium dioxide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7446-08-4
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 7446-08-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons