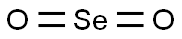Selenium dioxide: properties, applications and safety
General Description
Selenium dioxide is a colorless, pungent solid with a molecular weight of 110.96 g/mol, subliming at room temperature and soluble in water and organic solvents. It has a V-shaped structure and acts as an oxidizing agent, commonly used in organic synthesis and the production of selenium metal. Selenium dioxide is also employed in industries such as photography, glassmaking, and pharmaceuticals. However, it is toxic, requiring careful handling and protective measures to avoid health risks like respiratory irritation and selenosis. Its carcinogenic potential is currently not classifiable by IARC due to insufficient evidence.

Figure 1. Selenium dioxide
Properties
Selenium dioxide is a chemical compound with a molecular weight of 110.96 grams per mole. It is a colorless solid that sublimes readily at room temperature, with a characteristic pungent smell similar to that of sulfur dioxide. Selenium dioxide has a melting point of about 340°C and a boiling point of 315°C, though it sublimes easily at lower temperatures. As an oxidizing agent, SeO2 is often used in organic synthesis, particularly in the oxidation of ketones to carboxylic acids. The compound is also notable for its role in the preparation of selenium metal through reduction processes. In terms of structure, selenium dioxide possesses a V-shaped molecular geometry, akin to sulfur dioxide, with selenium at the center bonded to two oxygen atoms with double bonds, and the bond angle is approximately 120 degrees. Selenium dioxide is soluble in water, where it forms selenous acid (H2SeO3), which is weakly acidic. It is also soluble in organic solvents, which enables its use in organic reactions. It should be handled with caution, using appropriate protective equipment and under proper ventilation. Environmental exposure to selenium compounds must be monitored due to their potential toxicity to wildlife and humans. 1
Applications
Selenium dioxide is a versatile compound with multiple applications across various industries. Primarily, it serves as an important reagent in the synthesis of other selenium compounds due to its reactivity. In the field of photography, Selenium dioxide is used as a toner during the photographic development process, contributing to image quality and stability. Another significant application of selenium dioxide is in the glass manufacturing industry, where it is added as a colorant to impart a red hue to glass products. Additionally, selenium dioxide finds use in organic synthesis, where it acts as an oxidizing agent. It plays a role in the chemical analysis of alkaloids and is also utilized as an antioxidant in lubricating oils and a catalyst in certain reactions. In metal processing, Selenium dioxide is employed in etching baths, the creation of decorative colors on metals, and in providing corrosion protection for magnesium alloys. Furthermore, it is involved in the pharmaceutical industry for the synthesis of drug products, including the manufacture of cortisone and niacin. These varied applications highlight the chemical's importance in both industrial processes and consumer products. 2
Safety
Selenium dioxide is a chemical compound that, while essential in trace amounts for proper cellular function in many organisms, can pose health risks when exposure levels exceed the body's ability to metabolize and safely incorporate it. As a compound containing selenium, Selenium dioxide shares some toxicological properties with other selenium compounds. Acute exposure to high levels of selenium, such as from Selenium dioxide, could potentially disrupt cellular respiration by inactivating sulfhydryl enzymes necessary for oxidative reactions, leading to toxicity. Chronic exposure, on the other hand, might result in selenosis, a condition characterized by symptoms including hair loss, nail brittleness, and neurological issues. The carcinogenic potential of selenium compounds, including Selenium dioxide, has been classified as Group 3 by the International Agency for Research on Cancer (IARC), meaning it is not classifiable as to its carcinogenicity to humans due to insufficient evidence. However, given the potential for selenium compounds to generate reactive oxygen species through redox reactions with tissue thiols, there remains a theoretical risk of oxidative stress-related cellular damage. Therefore, handling Selenium dioxide requires caution, adherence to safety guidelines, and control of exposure to prevent acute or chronic selenium toxicity. 3
Reference
1. Selenium dioxide. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 24007.
2. SELENIUM DIOXIDE. Hazardous Substances Data Bank, Hazardous Substances DataBank Number: 677.
3. Selenium dioxide. Toxin and Toxin Target Database, Accession Number: T3D1819.
Related articles And Qustion
Lastest Price from Selenium dioxide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7446-08-4
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 7446-08-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons