D-Mannose: Overview, Clinical Applications in Urinary Tract Infections and Adverse Events
General Description
D-Mannose, a natural sugar, shows promise in preventing and treating urinary tract infections by inhibiting bacterial adhesion to the urothelium. It functions by blocking FimH adhesins on bacterial fimbriae, reducing the risk of UTIs caused by various uropathogens. Studies have demonstrated the effectiveness of D-Mannose in managing UTIs, both alone and in combinations, with positive outcomes in symptom improvement and UTI recurrence reduction. Adverse events are minimal, with diarrhea being the most common, but generally mild and well-tolerated. Compared to nitrofurantoin, D-Mannose has a lower risk of adverse events, making it a potentially safer option for UTI management.

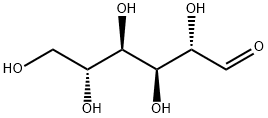
Figure 1. D-Mannose
Overview
D-mannose is emerging as a promising nonantibiotic approach for preventing urinary tract infections. It is a monosaccharide that is metabolized and excreted in urine, exerting its effects by inhibiting bacterial adhesion to the urothelium. As a simple sugar, D-mannose plays a crucial role in human metabolism through the glycosylation of proteins. Specifically, it functions by binding and blocking FimH adhesins located on the tip of type 1 bacterial fimbriae. This action makes D-mannose a competitive inhibitor against bacterial adhesion to the receptors of urothelial cells. Notably, type 1 pili, which are targeted by D-mannose, are present in various uropathogens including Escherichia coli, Klebsiella pneumoniae, Shigella flexneri, Salmonella typhimurium, Serratia marcescens, and Enterobacter cloacae. Consequently, D-mannose has the potential to prevent the adhesion of multiple uropathogens to the urothelium, thereby reducing the risk of UTIs. 1
Clinical Applications in Urinary Tract Infections
D-Mannose is a natural sugar that has been studied for its potential in preventing and treating urinary tract infections (UTIs). Several research studies have explored the effectiveness of D-Mannose in various formulations and combinations for managing UTIs in different patient populations. In a study by Domenici et al in 2016, D-Mannose alone was tested in 43 women for the treatment of acute UTIs and the prevention of recurrences. The results showed a significant improvement in symptoms after D-Mannose administration, and patients who received D-Mannose for prophylaxis had a longer time to UTI onset compared to those who did not receive treatment. Phe et al conducted a study in 2017 involving patients with multiple sclerosis and a history of recurrent UTIs. Participants were treated with D-Mannose powder and showed a decrease in the number of monthly UTIs with excellent compliance and no reported adverse effects. Marchiori et al in 2017 evaluated the effectiveness of a combination of N-acetylcysteine, D-Mannose, and a Morinda citrifolia fruit extract in reducing the persistence of recurrent UTIs in breast cancer survivors. The study showed a significant reduction in bacteria-positive urine cultures and improvement in urinary symptoms compared to antibiotic therapy alone. In a randomized study by Genovese et al in 2018, different formulations of D-Mannose combined with other compounds were compared in women with acute UTIs and recurrent cystitis. Patients in certain groups had a lower incidence of recurrent cystitis episodes during treatment and follow-up. Del Popolo and Nelli explored a combination of D-Mannose, salicin, and Lactobacillus acidophilus in patients with recurrent symptomatic cystitis, including those with neurogenic bladder. Significant improvement in symptoms was observed in both groups, with a reduction in daily frequency and incontinence episodes. Overall, these studies suggest that D-Mannose, either alone or in combination with other compounds, can be effective in preventing and treating UTIs in various patient populations, with promising results in terms of symptom improvement and UTI recurrence reduction. 1
Adverse Events
D-Mannose is generally well tolerated, with minimal reported adverse events. The most common adverse event associated with its use is diarrhea, occurring in approximately 8% of patients who received a dosage of 2 grams of D-Mannose for at least 6 months. However, these instances are typically mild and do not necessitate discontinuation of treatment. In a comparative study, patients administered D-Mannose exhibited a significantly lower risk of adverse events compared to those given nitrofurantoin (RR 0.276, 95% CI 0.132–0.574, p = 0.0001). This suggests that D-Mannose may be a safer option in terms of adverse event profile when compared to nitrofurantoin. 2
Reference
1. De Nunzio C, Bartoletti R, Tubaro A, Simonato A, Ficarra V. Role of D-Mannose in the Prevention of Recurrent Uncomplicated Cystitis: State of the Art and Future Perspectives. Antibiotics (Basel). 2021; 10(4): 373.
2. Kranj cec B, Pape D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: A randomized clinical trial. World J Urol. 2014, 32: 79–84.
Related articles And Qustion
See also
Lastest Price from D-Mannose manufacturers

US $0.00-0.00/g2025-06-06
- CAS:
- 3458-28-4
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 10000tons

US $10.00/Kg2025-04-24
- CAS:
- 3458-28-4
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton