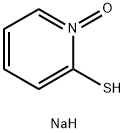
Sodium Pyrithione: Toxic Effects, Modes of Action and Mechanism of Action
Sodium pyrithione is used as a broad spectrum biocideespecially against fungi and gram positive and gram negative bacteria in metal-working fluids (boring and cutting oils, up to0.5 % in the concentrate) (Olin Corporation 1989f; Tenenbaum and Opdyke 1969), in the rubber industry(Wallhäusser 1984) and paint industry (dispersion paints,0.05 %–0.2 %) (Clayton and Clayton 1981), and in cosmetics which are rinsed off, such as shampoos and wash lotions forthe skin, in concentrations of 0.5 % (Lüpke and Preusser1978).

Toxic Effects and Modes of Action
Independent of the exposure route, sodium pyrithione is of low toxicity. The typical symptom of intoxication in rats, mice and rabbits given single or multiple doses of the substance is reversible paralysis of the rear extremities. This effect is not seen in monkeys or dogs. In both these species effects on the pupillary reflex and photophobia were observed. Irreversible eye damage, however, has been seen only in species which have a tapetum lucidum, for example, the dog.
Sodium pyrithione is readily absorbed from the gastrointestinal tract and through the intact skin. The substance is excreted rapidly in the form of urinary metabolites.
Applied to rabbits, the substance causes slight irritation of the skin and eyes. Brief contact with aqueous solutions containing less than 1 % sodium pyrithione produced no effects in animals or man; sensitization could not be demonstrated.
Reproductive toxicity is not observed, either after dermal application to rats or rabbits or after oral administration; to rats. Embryotoxicity develops in rats but not in rabbits after maternally toxic doses of sodium pyrithione.
Genotoxic effects of sodium pyrithione could not be demonstrated in theSalmonella mutagenicity test, in the HPRT (hypoxanthine guanine phosphoribosyl transferase) test or in the test for DNA repair in rat hepatocytes. However, because the substance is cytotoxic, only low concentrations could be tested. Negative results were also obtained in vivo in the micronucleus test.
Sodium pyrithione is not carcinogenic either after dermal application to mice or after oral administration to rats.
Mechanism of Action
The mechanism of the neurotoxicity of sodium pyrithione has not been studied but such investigations have been carried out with zinc pyrithione.
The biocidic action of sodium pyrithione is probably a result of a number of factors:
1. inhibition of alcohol dehydrogenase (Cotton 1963),
2. disturbance of proton gradients in cell membranes (Chandler and Segel 1978),
3. the action of the substance as an antimetabolite of pyridoxal (Chandler and Segel1978, Cooney 1969)
4. production of oxygen radicals during formation of iron complexes (Albert 1981).
The eye damage seen in animals like dogs which have atapetum lucidumis probably a result of destruction of this epithelium, which contains high levels of zinc, either by theformation of complexes with the zinc or by adverse effects on the enzymes of the tapetum(Delahuntet al.1962). Vitamin B6 deficiency has also been discussed in this context because the symptoms of eye damage are similar to those caused by deficiency of this vitamin. A mechanistic explanation of the effect has, however, not been offered(Moeet al.1960).
You may like
Related articles And Qustion
Lastest Price from Sodium Pyrithione manufacturers

US $0.00/KG2025-04-21
- CAS:
- 3811-73-2
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 500kg/month

US $9.80/KG2025-04-21
- CAS:
- 3811-73-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 tons