Applications of Titanocene dichloride
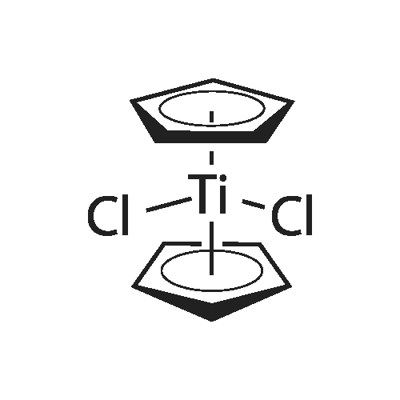
Titanocene dichloride, or dicyclopentadienyl titanium dichloride is (η5-C5H5)2TiCl2 (commonly abbreviated as Cp2TiCl2); this metallocene is widely used in organometallic and organic synthesis both as a reagent and as a catalyst. It is exists as a bright red solid, forming acicular crystals when crystallized from toluene. Cp2TiCl2 does not adopt the typical "sandwich" structure like ferrocene due to the 4 ligands around the metal centre, but rather takes on a distorted tetrahedral shape.

Applications
Hydrogenation of rubbers: Titanocene Dichloride and derivatives are used as hydrogenation catalyst for rubbers, like SBS and SEBS, to improve heat stability and resistance to ozone/oxidation.
Polymerization: Titanocene Dichloride can be used with polymethylaluminoxane as a catalyst for metallocene type olefin polymerizations.
Organic synthesis: Titanocene Dichloride is a useful reagent for a wide variety of synthetic transformations. It can be used with Grignard reagents for the reduction of aryl and vinyl halides; with magnesium for the reduction of organic halides, azo compounds, haloketones, and haloesters ; with sodium for the reduction of aliphatic aldehydes, esters, and epoxides; for the reductive decyanation of alkyl nitriles; and the reduction of olefins.
Titanocene Dichloride has been used with alkylaluminum compounds for the alkylation of α-olefins and alkynylsilanes. It can be reacted with trimethylaluminum to form Tebbe reagent, which is used to transform a carbonyl into a methylene group. Nouryon can further convert Titanocene Dichloride into derivatives required by the customer, e.g. by substituting the Cl-atoms by other groups or by replacing the cyclopentadienyl rings with substituted cyclopentadienyl rings.
Preparation
Cp2TiCl2 continues to be prepared similarly to its original synthesis by Wilkinson and Birmingham:
2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl
The reaction is conducted in THF. Work-up entails extraction into chloroform/hydrogen chloride and recrystallization from toluene. In the original literature, the structure was poorly understood. Each of the two Cp rings are attached to Ti(IV) through all five carbon atoms. In organometallic chemical jargon, this bonding is referred to as η5 (see hapticity).
You may like
Related articles And Qustion
See also
Lastest Price from Titanocene dichloride manufacturers

US $0.00-0.00/kg2025-10-22
- CAS:
- 1271-19-8
- Min. Order:
- 1kg
- Purity:
- ≥99.0%
- Supply Ability:
- 60tons

US $0.00/kg2025-03-03
- CAS:
- 1271-19-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS