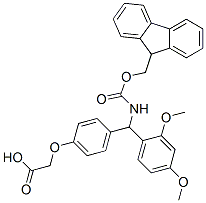
4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid: Synthesis, bioactivity and toxicity
![4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid.jpeg Article illustration](/NewsImg/2023-03-08/6381390811818001541519545.jpg)
4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid can be sy nthesized by single step according to the previous work [1]. Benzhydrylamine copolystyrene-1% divinylbenzene cross-linked resin (10.0 g, 9.3 mequiv, 100-200 ASTM mesh, Advanced ChemTech) was swelled in 100 mL CH2Cl2, filtered and washed successively with 100 ml each of CH2Cl2, 6% DIPEA/CH2Cl2 (two times), CH2Cl2 (two times). The resin was treated with p-((R, S)-α-(1-(9H-fluoren-9-yl)-methoxyformamido)-2,4-dimethoxybenzyl)-phenoxyacetic acid (Fmoc-Linker) (7.01 g, 13.0 mmole), N-hydroxybenzotriazole (2.16 g, 16.0 mmole), and diisopropyl-carbodiimide (2.04 ml, 13.0 mmol) in 100 mL 25% DMF/CH2Cl2 for 24 hours at room temperature. The resin was filtered and washed successively with 100 ml each of CH2Cl2 (two times), isopropanol (two times), DMF, and CH2Cl2 (three times). A Kaiser Ninhydrin analysis was negative. The resin was dried under vacuum to yield 16.12 g of Fmoc-Linker-BHA resin. A portion of this resin (3.5 mg) was subjected to Fmoc deprotection and quantitative UV analysis which indicated a loading of 0.56 mmol/g.
The 4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid can be analyzed by the method of Time-of-flight secondary ion mass spectrometry (TOF-SIMS) according to the previous work [2]. Briefly, crown samples analyzed by TOF-SIMS were cut out directly from the pins with a razor blade to obtain thin slices, which were then immediately placed on a stainless-steel grid. TOF-SIMS measurements were performed on a TRIFT I spectrometer (PHI-Evans). Spectra were recorded using a pulse (1 ns, 12 kHz) liquid metal source (69Ga, 15 keV) operating in the bunched mode of operation in order to provide good mass reolution (m/δm = 2000 measured at m/z 43). Owing to large charge effects on such insulating materials, charge compensation was needed for all samples and was achieved by a pulsing electron flood (Ek = 20 eV) at a rate of one electron pulse per five ion pulse. The analyzed surfaces were squares of 100 × 100 μm. All positive and negative spectra were acquired in 10 min with a fluence of less than 1012 ions cm-2, ensuring static conditions on the sample slices.
Bioactivity
Toxicity
As a preliminary assessment of toxicity, the viability assays for the 4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid which inhibited ALR2 to greater than 50% at 50 μM [3]. The
[2]Aubagnac et al. Application of time-of-flight secondary ion mass spectrometry to in situ monitoring of solid-phase peptide synthesis on the Multipin (TM) system. Journal of Mass Spectrometry, 1998, 33(11): 1094-1103.
[3]Wang et al. Discovery of New Selective Human Aldose Reductase Inhibitors through Virtual Screening Multiple Binding Pocket Conformations. Journal of Chemical Information and Modeling, 2013, 53(9): 2409-2422.
[4]Kador, P. F.; Kinoshita, J. H.; Sharpless, N. E. Aldose reductase inhibitors: a potential new class of agents for the pharmacological control of certain diabetic complications. J. Med. Chem. 1985, 7, 841? 849.
[5]Kinoshita, J. H.; Nishimura, C. The involvement of aldose reductase in diabetic complications. Diabetes Metab. Rev. 1988, 4, 323?337.
You may like
Related articles And Qustion
See also
Lastest Price from Rink Amide Linker manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 145069-56-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T+

US $100.00-80.00/kg2025-03-07
- CAS:
- 145069-56-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200Tons