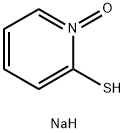
The prevention or reduction of discoloration of sodium pyrithione
Description
Zinc pyrithione [also known as zinc pyridine-2-thiol-N-oxide or bis[1-hydroxy-2(H) pyridinethionato]-zinc] is an excellent biocide. It has been employed as a broad-spectrum antimicrobial agent and preservative in metal working fluids, plastics, and cosmetics. Its principal uses are as an antidandruff agent in hair products or as a preservative in various cosmetics and toiletries. Sodium pyrithione [also called the sodium salt of 1-hydroxy-2-pyridinethione, sodium pyridine-2-thiol-N-oxide, or 2-pyridinethiol-1-oxide, Na salt] is also employed as a preservative in various cosmetics and toiletries.
Zinc pyrithione may be made by reacting 1-hydroxy-2-pyridinethione or a soluble salt thereof with a zinc salt (e.g., ZnSO4) to form a zinc pyrithione precipitate. See U.S. Pat. No. 2,809,971, which issued to Bernstein and Losee on Oct. 15, 1957. Generally, the sodium pyrithione is employed as the precursor of zinc pyrithione.
Since the esthetics of cosmetics and toiletries normally require certain desirable colors, and the formulators of such products go to great lengths to achieve specific color effects, any ingredient which varies very much from white or colorless may make the colorant formulators' task very difficult.
Process for the prevention or reduction of discoloration of sodium pyrithione
An aqueous solution of unpurified sodium pyrithione (13% by weight, 390.2 grams solution, 0.34 moles of active compound) containing 5.1 grams of a surfactant was charged into a 1000 ml round bottom flask equipped with stirrer, thermometer, heating mantle and addition funnel. The Gardner-Hellige Varnish Color Scale1 reading of this solution was 10.
Solid sodium bisulfite (3.6 grams, 0.034 mole) was then added to the flask at ambient temperature (about 20°-25° C.). The Gardner-Hellige Varnish Color reading was then measured to be 8. The solution was heated up to 95° C. during a period of 20 minutes. The solution appeared to darken slightly after this heating.
An aqueous solution of ZnSO4 (9.7% by weight ZnSO4, 270.0 grams, 0.163 moles ZnSO4) was added to the heated flask through the addition funnel. A precipitate made up of substantially zinc pyrithione was formed. This precipitate was removed from the reaction mixture by filtration.
The Hunter color2 values of this filter cake were measured to be as L=94.5, a=-5.4, and b=6.0. Calculated whiteness3 from these Hunter color values was 56.5 (as compared to MgO=100).
Related articles And Qustion
See also
Lastest Price from Sodium Pyrithione manufacturers

US $0.00/KG2025-04-21
- CAS:
- 3811-73-2
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 500kg/month

US $9.80/KG2025-04-21
- CAS:
- 3811-73-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 tons