Biological functions and synthesis of Aldosterone
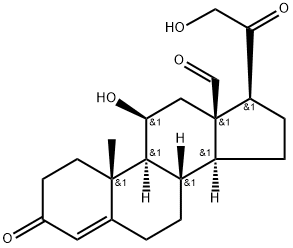
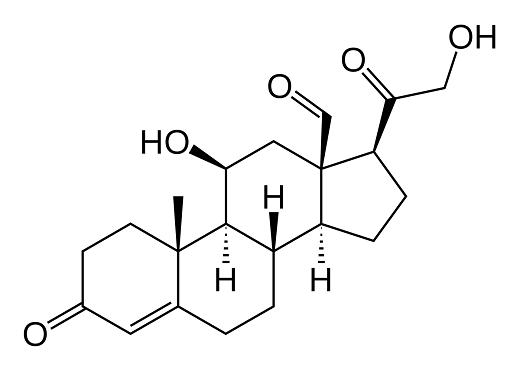
Structure
Structural features In 1954, the structure of aldosterone was reported.Aldosterone is synthesized from 18-hydroxycorticosterone by aldosterone synthase (CYP11B2). The production of aldosterone is regulated at the two critical enzyme steps: (1) first in its biosynthetic pathway (the conversion of cholesterol to the pregnenolone cholesterol side chain cleavage enzyme) and (2) the conversion of corticosterone to aldosterone by aldosterone synthase.
The steroidogenic acute regulatory protein (StAR) controls the transport of cholesterol to the inner mitochondrial membrane where P450scc (CYP11A1) is located. CYP11A1 converts cholesterol to pregnenolone. Pregnenolone can be modified to 11-deoxycorticosterone by two enzyme activities: 3β-hydroxysteroid dehydrogenase (3β-HSD) and 21α-hydroxylase at the smooth endoplasmic reticulum. 11-deoxycorticosterone is converted to corticosterone by 11β-hydroxylase in the mitochondria in the zona fasciculata of the adrenal gland. In the zona glomerulosa of the adrenal gland, corticosterone is modified to 18-hydroxycorticosterone and then to aldosterone. This synthetic cascade is regulated by aldosterone synthase, CYP11B2. Aldosterone synthase is absent in other sections of the adrenal gland, and teleost fish lack aldosterone synthase (CYP11B2), suggesting that the dominant corticosteroid in fish is cortisol, although 11-deoxycorticosterone and progesterone have also been proposed.
Discovery
Simpson et al.developed a bioassay with high sensitivity for mineralocorticoid activity, and crystallized aldosterone (electrocortin) from beef adrenal glands.
Regulation of synthesis and release
A variety of factors modify aldosterone secretion, such as the level of pituitary adrenocorticotropic hormone (ACTH), kidney angiotensin II, and the plasma concentration of potassium ion. ACTH acts as an enhancer of StAR synthesis via the activation of cAMP-dependent protein kinase A. Angiotensin II, which is converted from angiotensin I by the angiotensin-converting enzyme, is involved in the regulation of aldosterone. Angiotensin II stimulates the synthesis and release of aldosterone from the zona glomerulosa (renin-angiotensin system). High levels of serum potassium directly stimulate aldosterone secretion from the zona glomerulosa. An increase in dietary potassium induces an increase in aldosterone secretion, whereas potassium depletion causes a decrease.
Some animal studies show the physiological function for potassium in the regulation of aldosterone secretion.Thus, alterations in potassium balance as well as acute increments in serum potassium can stimulate the production of aldosterone.On the other hand, the atrial natriuretic peptide (ANP) is an inhibitory factor of aldosterone secretion. ANP secretion is increased in response to sodium and/or water loading, and it in turn inhibits aldosterone secretion.In humans, the ANP dosage induces the reduction of plasma renin and aldosterone concentrations and inhibits angiotensin II-induced aldosterone secretion.
Biological functions
Physiological actions Aldosterone achieves its physiological effects by controlling the transcription of specific genes activated by the mineralocorticoid receptor (MR) in target cells. Aldosterone has mineralocorticoid activity by controlling sodium homeostasis. Aldosterone regulates the transcription of the epithelial sodium channel and Na+ /K+ -ATPase subunit genes.Aldosterone promotes the reabsorption of sodium from renal tubules. In this way, aldosterone is a critical regulator of serum sodium.
Mechanisms of actions
Aldosterone acts in target cells by binding to and activating the MR. The aldosterone-MR complex binds to specific hormone response elements located at the gene promoter region, which leads to gene transcription. Human MR contains 984 amino acids. It has 57% amino acid identity with the human glucocorticoid receptor (GR) in the ligand-binding domain, and 94% in the DNA-binding domain.Nongenomic pathways are also a part of aldosterone-mediated biological actions via the nonclassical receptor and the membrane-bound MR. This nongenomic pathway, which is less characterized to date, involves numerous signaling pathways in the kidney, colon, vascular wall, and heart.
You may like
See also
Lastest Price from ALDOSTERONE manufacturers

US $0.00-0.00/KG2025-05-19
- CAS:
- 52-39-1
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS