Research on the drug formulation of famotidine aimed at prolonging gastric duration
Introduction
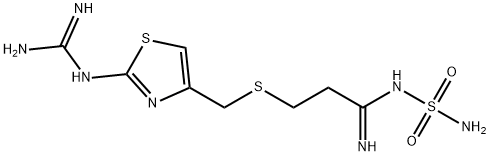
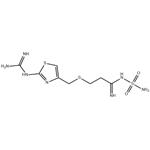
Famotidine (Figure 1) is the third generation of potent H2 receptor antagonist. Through uniting with the H2 receptor in the cell of stomach, famotidine manages to inhibit the secretion of gastric acid. It is mainly used for gastric, duodenal ulcer, reflux esophagitis, upper gastrointestinal bleeding and other diseases on clinical. It has high therapeutic effectiveness that is 20-fold of cimetidine and 7.5-fold of ranitidine, respectively. Although, these are many dosage forms to select such as tablets, orally disintegrating. tablets, oral suspensions, injections and powder, as well as fixed formulation of ibuprofen and famotidine nowadays, some problems have been existing in clinical application like short retentive time in the stomach, low concentration in parietal cell, short half-life and strong fluctuations of plasma concentration, so famotidine is fully able to no utilization. It is urgent to manufacture a novel formulation in order to meet the challenge from treatment of stomach diseases through gastric retention technology.

Famotidine Gastric Bioadhesive Microspheres
In He's study[1], a new famotidine gastric bioadhesive microspheres has been fabricated to solve the clinical problem and is significant to be convenient to patients, reduce side effects, and enhance value of the old drug. An excellent formulation and process with strong mucoadhesive performance was obtained by using ethyl cellulose as matrix material, carbopol 934P as bioadhensive agent, PEG 6000 as pore-foaming agent, polysorbate 80 as emulsifiers, liquid parafin as continuous phase and through emulsion-solvent evaporation method; Release in pH 1.0 solution fits zero-order equation; Carbomer showed obvious adhesion properties in four experiments in vivo or in vitro; Pharmacokinetic studies showed gastric bioadhensive microspheres with sustained release, relatively lower plasma concentration in vivo, no bioequivalence compared with conventional tablets, a significant correlation feature between in vivo and in vitro, higher relative bioavailability of approximate 130% for test formulation. The formulation showed highly gastric targeting.
Famotidine Floating Pellets/microspheres
Floating Drug Delivery System (FDDS), as a kind of potential dosage form, could improve the absorption of drugs with an absorption window in the stomach or in the upper small intestine.diminish the side effects, stable the blood concentration, reduce the administration frequency and improve the clinical effect, FDDS is desirable for drugs with the following properties: (a) Having an absorption window in the stomach or in the upper small intestine; (b) Stable and soluble in acid condition; (c)Gastric acid secretion inhibitor, (d) Having marked effect in stomach; (e) Low soluble or insoluble in intestine.
Yu etal. prepared famotidine to the floating dosage form[2]. In this investigation, extrusion-spheronization method was applied to prepare the light drug-loaded pellets with large amount of stearic acid to make the pellets float immediately after being put into the medium. Then, fluid-bed method was used to coat the core pellets with effervescent layer and entrapped layer which could keep the pellets float and sustained release of the drug. The finally floating pellets could float immediately after being put into the medium, keep floating more than 8 hours and sustained release the drug 8 hours.
HPLC method was developed to quantify the drug plasma concentration of rabbits, and the studies of pharmacokinetics were performed after oral administration of three formulations included famotidine floating pellets, famotidine sustained-release pellets (infloating pellets) and domestic famotidine tablets. The pharmacokinetic parameters of these three formulations were as below: T1/2 were 2.529±0.948, 2.553±1.584 and 1.468±0.738h; Tmax were 5.000±0,2.583±0.492 and 1.917±0.585 h; Cmax were 0.297±0.094, 0.140±0.023 and 0.397±0.148μg/mL. The relative bioavailability of sustained-release floating pellets was 175.8±36.7% compared with domestic tablets, and 49.3±25.1% for sustained-release pellets to domestic tablets. The famotidine floating pellets would extend the acting duration, reduce administration frequency and increase the bioavailability.
In addition, famotidine core pellets were prepared by Huang et al.[3]. The drug-loaded pellets which had a bulk density of less than 1g/cm3 because of containing large amount of ceraceous materials (stearic acid) were prepared by extrusion-spheronization technique. The micromeritic properties and floating ability of the core pellets were evaluated to optimize the formulation and preparation. Then, an inner effervescent layer (HPMC with NaHCO3) and an outer gas-entrapped layer (Eudragit®NE30D) were coated outside the core pellets to maintain the pellets float and release the drug more than 8 hours. The results showed that the obtained pellets had excellent features, such as the uniform size, which is 1.92mm of mean diameter, and the satisfactory roundness, which is 0.92 of aspect ratio. The pellets could float within 2min and keep floating for more than 24h upon 0.1mol/L hydrochloric acid.
Furthermore, the famotidine microspheres were prepared by the emulsion solvent evaporation method. Through examining the type and dosage of different carriers, oil phase, composition of emulsifier, the volume and pH, temperature of aqueous phase and the amount of three citric acid ethyl ester, EUDRAGIT s100 was finally confirmed as the drug carrier. The ratio of the carrier to the drug is 8:1. The ratio of ethanol to dichloromethane is 10:6. Based on the following matches of different experimental conditions, the content of three citricacid ethl ester being 0.2g, the temperature beings 40℃, the volume beings 150ml, and the emulsifier being PVA124, the results showed that the yield is 47.66%, drug loading capacity is 7.70% , the average entrapment efficiency is 69.37%, the floating behavior is good and 90% microspheres is still floating on the surface within 24h. Both pellets and microspheres have good slow-releasing effect, which is equal to bio-tech drugs.
Conclusion
Some novel famotidine gastric bioadhesive microspheres or floating pellets/microspheres would meet the challenge from clinical requirements. They could achieve the primal purposes of both in vitro and in vivo, that is extend the acting duration, reduce administration frequency and increase the availability.
References
[1] He Xt. Study on Famotidine Gastric Bioadhesive Microspheres[D].Shandong University,2014.
[2] Yu Py. Studies on Famotidine Sustained-Release Floating Pellets[D].Shenyang Pharmaceutical University,2009.
[3] Huang Yy. Studies on Famotidine Gastric floating preparations[D].Zhejiang University of Technology,2013.
You may like
Related articles And Qustion
Lastest Price from Famotidine manufacturers
US $0.00/kg2025-10-26
- CAS:
- 76824-35-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $5.00-0.50/KG2025-05-07
- CAS:
- 76824-35-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS