Cyclen: chemical synthesis and applications of this macromolecule
Chemical synthesis route
Cyclene (1,4,7,10-tetraazacyclododecane) is a frequently used starting material in the production of macrocyclic complexing agents and is mainly used in the area of nuclear resonance tomography as a ligand for gadolinium. Two preparations are already commercially available with ProHance.RTM. of Bristol-Myers-Squibb and Dotarem.RTM. of Guerbet. Special research and development projects also use cyclene as a starting material. There is therefore a need for an easy and economical process for the production of this educt.
A profitable process should use raw materials that are as reasonably priced, as environmentally safe and as easily accessible as possible. It has been found that a process for the production of cyclene in a single-pot process, triethylenetetramine is reacted with 40% glyoxal at 20 DEG C. to 80 DEG C. in a polar, protic solvent, preferably methanol, ethanol, isopropanol, butanol, glycol, water or mixtures thereof, especially preferably ethanol, within 4 to 40 hours, preferably 15 to 20 hours; after the solvent has been removed, the intermediate tricyclic compound that is thus formed is alkylated to the two secondary amine-nitrogens with a 1,2-difunctionalized alkylating agent X(CH2)2 X, in which X stands for a nucleofuge group, preferably with 1,2-dibromoethane, 1,2-dichloroethane, 1,2-ditosylethane, 1,2-dimetylethane or 1,2-dioodoethane, especially preferably with 1,2-dichloroethane in a polar aprotic solvent, preferably in N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMAC), N-methylpyrrolidone (NMP), tetramethyl urea, formamide or dimethylpropylene urea (DMPU), especially preferably in DMF, optionally in the presence of an auxiliary base, preferably sodium carbonate, potassium carbonate, calcium carbonate, sodium bicarbonate, potassium hydrogen carbonate, magnesium carbonate, magnesium hydrogen carbonate, lithium hydroxide or lithium carbonate, especially preferably without an auxiliary base, at 20 to 120 DEG C., preferably 30 to 70 DEG C., within 2 to 24 hours, preferably 6 to 10 hours; after the solvent has been removed, the thus obtained condensation product is treated with hydrazine hydrate in a polar protic solvent, preferably methanol, ethanol, isopropanol, butanol, glycol, water and/or mixtures thereof, especially preferably ethanol, at a pH of 3 to 6, preferably 3 to 4, 12 to 48 hours, preferably 25 to 35 hours, at reflux temperature; then the cyclene is released from the cyclene salt by adding a base, preferably sodium hydroxide, potassium hydroxide, calcium hydroxide or a basic ion exchanger, especially preferably sodium hydroxide and potassium hydroxide, and after the reaction solution is evaporated to the dry state, it is isolated, surprisingly enough achieves the above-mentioned object. The isolation of the cyclene is preferably carried out by crystallization from toluene, trifluoromethylbenzene or diethoxymethane, whereby the latter is especially preferred.[1]

The following example is used for a more detailed explanation of the synthesis procedure:Dowex TM 1 × 8 200-400 mesh strong basic type I anion exchange resin (Cl type) was placed in a 300 mL beaker and dissolved in 1 M HCl. After filtering this with Nutche, the obtained filtrate was dissolved in distilled water and poured into a chromatographic tube filled with cotton and sea sand. Followed by adding 1 M HCl until the pH of the droplet became 1, the pH was adjusted to 5 with distilled water. Then add 1 M NaOH until the pH reaches 12 and then the pH was adjusted to 7 using distilled water. A solution prepared by dissolving 1,4,7,10-tetraazacyclododecane tetrahydrochloride (1.50 g, 4.72 mmol) in the smallest amount of distilled water was put therein, and droplets having a basic pH were collected. This solution is concentrated on a rotary evaporator and Vacuum drying gave a yellow solid. Dissolve this in CHCl3, add Na2SO4 to dehydrate it, filter it with Nutche, wash it with a small amount of CHCl3, collect the filtrate, and concentrate it with a rotary evaporator vacuum dried and left in the refrigerator to give a yellow solid (0.772 g, Yield 95%).
Applications
solar cells
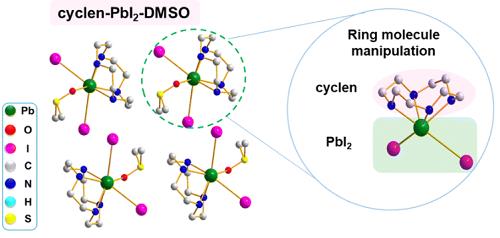
Although perovskite solar cells (PSCs) have been developed rapidly, the poor quality of perovskite films and difficulty in the scalable fabrication under air conditions hinder the improvement of PSC performance. Researchers introduced cyclen to manipulate high-quality film fabrication. They, for the first time, captured a key intermediate, cyclen-PbI2-DMSO, which retarded the crystallization process, promoted the carrier lifetime, and decreased non-radiative recombination. According to DFT calculations and experimental characterization, the cyclen interacted well with Pb2+ ions to manipulate perovskite film crystallization and reduce defect density. Consequently, the small-area device with a power conversion efficiency up to 24.71% was fabricated and could still maintain 90% of its original PCE over 1600 h at 85 °C in an N2 atmosphere without encapsulation. Additionally, the constructed 36 cm2-area cyclen modules yielded an efficiency of 20.08% via the auto-blade coating process under air conditions.
Molecule regulation is often used to manipulate Pb-related defects in a perovskite with functional O, S, and P atoms to donate the lone pair of electrons to Pb2+ ions. As a typical molecule, crown ether has been recognized as modifiers or stabilizers in PSCs with a unique ring structure and abundant oxygen chelating with Pb2+ ions to improve the efficiency and stability. Despite the enhancement by crown ethers, most of the devices still suffer from large Voc deficit and unsatisfactory PCE values.
In the above work, a ring molecule containing multi-nitrogen atoms, cyclen, is introduced into triple-cation CsFAMA, double-cation CsFA or single-cation FA perovskite precursors to manipulate the uniformity and crystallization of perovskite films. The N–H bond in cyclen can easily interact with Pb2+ ions to generate a stable Pb–N bond in a novel zero-dimensional intermediate (cyclen-PbI2-DMSO) to reduce the PbI2 species. Cyclen not only effectively facilitates dense and high-quality film growth but also simultaneously reduces bulk/surface defects, thus accordingly improving the operation stability of the PSCs. Employing cyclen, we achieved a champion PCE of 24.71%, which is the highest PCE value among PSCs-manipulated ring-structure molecules. The unencapsulated devices maintain 90% over 1600 h at 85 °C and retain 91% of their initial PCE over 200 h under light illumination in N2 atmosphere. Moreover, we successfully fabricated the 36 cm2-area modules with cyclen regulation with the efficiency of 20.08% by the auto-blade coating process. The results demonstrate the great potential of this novel cyclen additive to promote the future commercialization of PSCs.
DNA delivery
Gene therapy has been expected to be a powerful approach to the treatment of genetic and acquired diseases. Generally speaking, gene transfection vectors commonly used in gene therapy mainly fall into two categories: viral and non-viral. In early days, viral vectors such as adenovirus and retrovirus have achieved some success particularly in cancer gene therapy for their relatively high transfection efficiency (TE). However, their disadvantages such as adverse immunogenic responses, oncogenic effects, potential mutagenesis and high cost limited their applications. People thus try to find a more efficient and safe delivery system. For their easy preparation and modification, safety, and reproducibility, non-viral vectors attracted the attentions of scientists. As a polyamine compounds, 1, 4, 7, 10-tetraazacyclododecane (cyclen) has unique cyclic structure. The amino groups, which are easy to be modified, have wide range of pKa values. In our previous studies, we have demonstrated the potential of cyclen-based gene delivery systems.
On the other hand, lipids with monounsaturated hydrophobic chain(s) (e.g., oleyl group) might facilitate the transfection, since they could facilitate membrane disruption and DNA escape from endosome by increasing the membrane fluidity.
Many oleyl-contained lipids have been proved to be efficient delivery materials, and some commercially available transfection reagents also have oleyl moiety in their structures.
Besides the cationic headgroup and hydrophobic tails, the linking group also plays important role in the vector structure. Bajaj et al. showed that the length of polymethylene or oxyethylene spacers would influence the gene delivery properties of aromatic backbone based gemini lipids. Kedika and Patri found that the lipid with symmetrical backbone might give much higher TE than those with asymmetrical backbone structure. More recently, Niyomtham et al. revealed that the spermine-based cationic lipids with diverse central core structures had much different TEs. Scientists design and synthesize a series of cationic lipids with cyclen as cationic headgroup and oleyl as hydrophobic tails. These lipids have different linking group structures, which would help us to study their influence on the transfection behavior of the liposome/DNA complexes (lipoplexes). The structures of lipids studied here are listed: Aspartic acid, glutamic acid, diethanolamine, diethylenetriamine, serinol and 3-amino-1, 2-propanediol were used as linking group for lipids L1–L6, respectively. Their interactions with plasmid DNA together with their structure–activity relationship as non-viral gene delivery vectors were investigated.
References
[1] SCHERING AG. (2000). Process for the production of cyclene. US Patent 6,156,890.
[2] Wang, B., Li, Y., Zhang, Y., & Jiang, X. (2015). Cyclen-based double-tailed lipids for DNA delivery: Synthesis and the effect of linking group structures. Bioorganic & Medicinal Chemistry Letters, 25(24), 5849-5854.
[3] Yang, Y., Yuan, L., Chang, Q., Yang, X., Tang, X., Wan, Z., Du, J., Wei, H., Liu, C., Guo, P., Liu, Z., Chen, R., & Wang, H. (2024). Cyclen molecule manipulation for efficient and stable perovskite solar cells. Journal of Materials Chemistry A, 12, 13212-13218.
See also
Lastest Price from Cyclen manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 294-90-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100mt/year

US $120.00/kg2025-04-15
- CAS:
- 294-90-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton