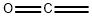Production of Ketene
Ketene [463-51-4], CH2=C=O, C2H2O, Mr 42.02, is a colorless, toxic gas with a characteristic odor; it is unstable at room temperature and atmospheric pressure. Ketene is very soluble in acetone and lower esters; it is only slightly soluble in hydrocarbons and halogenated hydrocarbons.

Chemical Properties
Ketene is an extremely reactive and unstable compound: it dimerizes to diketene and polymerizes very quickly. The 4-methyleneoxetan-2- one structure of diketene was established in 1952 by X-ray diffraction. Under the conditions for the industrial production of diketene, less than 5 % of ketene dimerizes to 1,3-cyclobutanedione in its monoenolic form, which in turn is further acetylated. It can only be stored at liquid nitrogen temperature. To prevent polymerization, reactions with ketene are best conducted under reduced pressure. If this is not possible, the reaction vessel should be directly connected to the ketene source or compressor.
Production
Ketene is produced by pyrolysis of acetic acid [64-19-7], which, although endothermic, is quite an efficient process. Decomposition products formed in the pyrolysis of acetic acid to ketene include methane, hydrogen, carbon monoxide, carbon dioxide, and ethylene. Higher acids can also be cracked to the corresponding ketenes, although the efficiency of conversion decreases as the chain length increases.