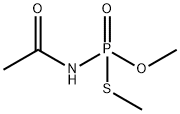
A widely used pesticide: Acephate
Description
Acephate is an organic compound with the chemical formula C4H10NO3PS. The pure product of acephate is a white crystalline phase and has a pungent odor. This substance is readily soluble in water, DMSO, and Chloroform. As a low-toxicity organophosphate insecticide, acephate has been widely used on food crops and no-food crops to control chewing and sucking insects.

Uses
This compound is a pesticide, and its metabolite methamidophos is highly toxic for mammals and birds; even so, it is one of the most used insecticides in pest control for agricultural and domestic use. As a systemic insecticide, this drug could effectively control various pests on ornamental plants, cotton, beans, and head lettuce as well as parasites on mammalians. After the acephate is applied to plants, it is absorbed by the roots or foliage of plants and transferred into the plants. When chewing and sucking insects feed on those plants, the acephate attacks the nervous system of insects and kills them. It is a good substitute because it is less toxic than methamidophos. Acephate is a class II “moderately hazardous” pesticide, but methamidophos is classified as a class IV “highly toxic” pesticide.
Toxicity effect
Acephate is highly water-soluble and can easily contaminate groundwater and soil, which is also easily absorbed by plants and accumulated in edible parts of plants. This pesticide has high to medium acute oral or medium inhalation toxicity to mammals. It will hurt the nervous and respiratory system, and cause eye and gastrointestinal problems in humans. People exposed to acephate have had nausea, diarrhea, abdominal cramps, shaking, sweating, rapid heart rate, dizziness, and/or confusion. Symptoms usually begin within minutes or hours after exposure. Pets might be exposed to acephate by eating granules from the ground.
Acephate is used in enormous quantities because of its high efficiency in agriculture and other industrial purposes, which leads this drug to remain in various environments for many years. In addition, Acephate toxicity affects both target and non-target organisms, thus leading to serious damage to the environment.
Therefore, it is a concerning issue to degrade and remove acephate in the plants and environment. Many studies have been done to degrade and remove acephate, most of which involve biodegradation method, acid hydrolysis, photocatalytic decomposition, electrolyzed water treatment, ozone method, supercritical carbon dioxide method, and γ-irradiation methods.
References
[1] Lin Z, et al. Degradation of Acephate and Its Intermediate Methamidophos: Mechanisms and Biochemical Pathways. Frontiers in Microbiology, 2020.
[2] Huang Y, et al. The mechanisms and process of acephate degradation by hydroxyl radical and hydrated electron. Saudi Journal of Biological Sciences, 2018; 25: 226-233.
You may like
Related articles And Qustion
See also
Lastest Price from Acephate manufacturers

US $0.00/kg2025-09-28
- CAS:
- 30560-19-1
- Min. Order:
- 100kg
- Purity:
- 98
- Supply Ability:
- 10000

US $0.00-0.00/KG2025-04-15
- CAS:
- 30560-19-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg