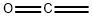Ketene: Applications in Organic Synthesis and its Health Hazards
General Description
Ketene, a reactive gas with diverse applications in organic synthesis, is known for its role in acetylating nucleophiles to produce esters and amides. Despite its utility, ketene poses significant health risks, with inhalation exposure leading to severe respiratory distress and lung abnormalities. Studies suggest similarities between ketene and phosgene in their mode of action, highlighting the importance of understanding and regulating ketene exposure, particularly in vaping products. Further research is crucial to elucidate ketene's effects, prevent adverse health outcomes, and ensure the safety of individuals using products containing this hazardous compound.

Figure 1. Ketene gas
Overview
Ketene, also known as ethenone, is a colorless and toxic gas characterized by a distinct "penetrating" odor. It exhibits solubility in a wide range of organic solvents but undergoes decomposition upon contact with water, resulting in the formation of acetic acid. The tautomer of ketene, ethynol, has been observed only through the photoisomerization of ketene within an argon matrix. German chemist Hermann Staudinger, a Nobel Prize laureate, made early discoveries regarding the ketene family of organic compounds in the early 20th century. Ketene is typically synthesized by pyrolyzing acetone, acetic acid, or acetic anhydride, or by treating acetyl chloride with a non-protic nucleophile. Its utility lies in its ability to acetylate nucleophiles, facilitating the synthesis of esters, amides, and other compounds that may not be readily achievable with alternative reagents. Ketenes played a crucial role in the synthesis of pioneering antibiotics such as penicillin and amoxicillin. 1
Applications in organic synthesis
Ketenes have been widely utilized in organic synthesis and the chemical industry for nearly a century. Initially employed for the production of acetic anhydride and cellulose acetate in the 1920s, ketenes are considered fundamental reactive intermediates in organic chemistry, alongside carbocations, carbanions, radicals, and carbenes. While the formation and function of other reactive intermediates have been extensively studied, the role of ketenes has been challenging to investigate due to their transient nature. Recent studies suggest that ketenes may influence selectivity in zeolite-catalyzed processes. Some findings propose a positive impact on selectivity, while others indicate potential catalyst deactivation through coke species formation. Resolving this discrepancy is crucial for advancing catalytic processes. In a notable 2016 study by Jiao et al., ketenes were detected during the conversion of syngas to light olefins using a composite catalyst. The presence of ketene was linked to enhanced selectivity for C2–C4 hydrocarbons, highlighting the significance of this intermediate in zeolite catalysis. 2
Health hazards
Ketene poses significant health hazards based on concerning toxicological studies. Inhalation exposure to ketene at concentrations ranging from 50 to 1000 parts per million (ppm) for as short as 10 minutes resulted in mortality within 0.8 to 16 hours across various animal species. In a study utilizing high-purity ketene (98-99%), monkeys exposed to concentrations as low as 12 ppm exhibited acute pulmonary congestion and alveolar edema. Additionally, mice exposed to 1 ppm for 14 days showed a 10% mortality rate. Human data on ketene toxicity are scarce, but a case report documented severe respiratory distress and lung abnormalities in a worker exposed to a concentrated mixture of ketene and crotonaldehyde. Ketene is believed to act similarly to phosgene, a chemical warfare agent, by acylating proteins and disrupting the blood-air barrier, leading to edema and inflammation. The mode of action of ketene aligns with the clinical presentations observed during the EVALI outbreak, where vaping-associated lung injuries were reported. Ketene's poor warning properties further exacerbate its risk, especially in the context of vaping, where users may be exposed to unknown concentrations through various devices and e-liquid compositions. Moreover, ketene's presence in unregulated vaping products, along with potential interactions with other compounds such as flavorings and diluents, underscores the need for further research and regulation in the vaping industry. Mechanistic insights into ketene's effects could inform preventive measures and aid in understanding the long-term health consequences, including DNA damage and carcinogenicity. Animal models may be instrumental in elucidating these mechanisms and assessing the impacts of vaping on lung health. Overall, comprehensive studies are essential to mitigate the health risks associated with ketene exposure, particularly in the context of vaping and e-cigarette use. 3
Reference
1. Ketene. American Chemical Society. 2016.
2. Chowdhury AD, Gascon J. The Curious Case of Ketene in Zeolite Chemistry and Catalysis. Angew Chem Int Ed Engl. 2018; 57(46): 14982-14985.
3. Attfield KR, Chen W, Cummings KJ, et al. Potential of Ethenone (Ketene) to Contribute to Electronic Cigarette, or Vaping, Product Use-associated Lung Injury. Am J Respir Crit Care Med. 2020; 202(8): 1187-1189.