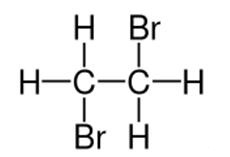
Related uses of 1,2-Dibromoethane
1,2-Dibromoethane [106-93- 4], BrCH2CH2Br (ethylene dibromide), is manufactured by the uncatalyzed addition of bromine to ethylene.

The commercial process is carried out in a glass-column reactor consisting of a lower packed section and an unpacked upper section containing a number of superimposed, highcapacity, coil heat exchangers. Liquid bromine is continuously added above the packed section while a slight excess of ethylene is continuously fed countercurrently from the bottom of the packed section. The exothermic reaction between ethylene and bromine occurs in the liquid phase on the surfaces of the cooling coils, and heat is removed at a rate sufficient to maintain a maximum temperature of 100℃ in this section of the column. Some reaction also occurs in the gas phase above the bromine feed where product is condensed and separated from the vent gas (ethylene, HBr, and inert material). As the crude liquid product passes downward through the packed section, it provides a contacting surface for rising ethylene to convert any residual dissolved bromine.
1,2-Dibromoethane is continuously withdrawn from the reactor into a hold-up tank where it is irradiated with ultraviolet light to eliminate minor amounts of unconverted starting materials. The technical-grade product is obtained in 99.5 % purity as a clear, colorless liquid in close to quantitative yield. A continuous process that claims lower corrosivity and high output of 1,2-Dibromoethane involves the countercurrent addition of bromine and ethylene plus the continuous addition of a cold concentrated aqueous solution of inorganic bromide, which functions as the heat exchange medium. A process that uses 1,2-Dibromoethane as the reaction medium is also reported to allow good control of the exothermic reaction between bromine and ethylene.
The largest market for 1,2-Dibromoethane is in gasoline. It acts as a scavenger for lead alkyls and prevents buildup of lead oxides in automobile engines. This use, which amounted to about 35 – 40 % of United States bromine consumption in 1981, has been declining since 1974 as a result of the lead phasedown mandated by the U.S. Environmental Protection Agency (EPA). Government agencies in many other countries are following this course of action to decrease the amount of lead in the atmosphere. The continued production of nonlead tolerant automobiles and conservation policies will more than likely result in further declines.
The other major application for 1,2-Dibromoethane is for soil and space fumigation, and the postharvest fumigation of fruit. It was used as a substitute for DBCP (1,2-dibromo-3-chloropropane), BrCH2CHBrCH2Cl [96-12-8], which was banned by the EPA in the late 1970s because workplace exposure led to azoospermia; it was applied to control pests, such as soil nematodes, rodents, and most species of insects. In late 1983 the EPA took emergency action to cancel the pesticide registrations for ethylene dibromide formulations used in most soil fumigation applications in the United States because of groundwater contamination. Some minor fumigation uses were allowed to continue, but the predominant volume of ethylene dibromide used in soil fumigation will cease.
Smaller amounts of 1,2-Dibromoethane are used as a reaction intermediate in the manufacture of other chemicals, such as the reactive flame retardant monomer, vinyl bromide [593-60-2]. Minor amounts are used as a highdensity, nonflammable solvent in a variety of applications.
Related articles And Qustion
Lastest Price from 1,2-Dibromoethane manufacturers

US $10.00-5.00/KG2025-06-27
- CAS:
- 106-93-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1.00/KG2024-10-11
- CAS:
- 106-93-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100000kg