Applications of 1,2-Dibromoethane
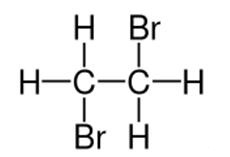
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula (CH2Br2). It is a colourless liquid. The vapour is stable, nonflammable and 3.5 times denser than air. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint sweet odor, detectable at 10 ppm. is used for the fumigation of stored grains, fruits, and vegetables. It is also used for soil disinfection against nematodes. As a fumigant ethylene dibromide is restricted in many countries, because of its carcinogenic potency. It does not appear to be teratogenic [1]. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive. 1,2-Dibromoethane was once used as a pesticide. In addition to being used to kill insects and other pests, 1,2-Dibromoethane was also added to gasoline. It is mostly man- made but it may be found naturally in the ocean in very small amounts.
Small amounts of 1,2-dibromoethane can be found in soil near hazardous waste sites. It can also be found on agricultural fields or in areas once used for farming. While 1,2-dibromoethane remains in groundwater and soil for a long time, it breaks down quickly in the air. Generally, environmental levels are very low. In the 1970s and early 1980s, 1,2-dibromoethane was used to kill insects and worms on fruits, vegetables and grain crops. It was also used to protect grass on golf courses and as an additive in leaded gasoline. Most of these uses stopped in 1984. 1,2-Dibromoethane is produced by the reaction of ethylene gas with bromine [2], in a classic halogen addition reaction:
CH2=CH2 + Br2→BrCH2-CH2Br
Historically, 1,2-dibromoethane was used as a component in anti-knock additives in leaded fuels. It reacts with lead residues to generate volatile lead bromides, thereby preventing fouling of the engine with lead deposits.
1,2-Dibromoethane has been used as a pesticide in soil and on various crops. The applications were initiated after the forced retirement of 1,2-dibromo-3-chloropropane (DBCP) [1]. Most of these uses have been stopped in the U.S. It continues to be used as a fumigant for treatment of logs for termites and beetles, for control of moths in beehives.
1,2-Dibromoethane has wider applications in the preparation of other organic compounds including those carrying modified diazocine rings [3] and vinyl bromide that is a precursor to some fire retardants [1].
In organic synthesis, 1,2-dibromoethane is used as a source of bromine to brominate carbanions and to activate magnesium for certain Grignard reagents. In the latter process, 1,2-dibromoethane reacts with magnesium, producing ethylene and magnesium bromide, and exposes a freshly etched portion of magnesium to the substrate [4].
1,2-Dibromoethane causes changes in the metabolism and severe destruction of living tissues. The known empirical LD50 values for 1,2-dibromoethane are 140 mg kg–1 (oral, rat), and 300.0 mg kg–1 (dermal, rabbit). 1,2-Dibromoethane is a known carcinogen, with pre-1977 exposure levels ranking it as the most carcinogenic substance on the HERP Index.
The effects on people of breathing high levels are not known, but animal studies with short-term exposures to high levels caused depression and collapse, indicating effects on the brain. Changes in the brain and behavior were also seen in young rats whose male parents had breathed 1,2-dibromoethane, and birth defects were observed in the young of animals that were exposed while pregnant. 1,2-Dibromoethane is not known to cause birth defects in humans. Swallowing has caused death at 40 mL doses [5].
It can be absorbed through the skin, lungs and gastro-intestinal tract. It appears to be metabolized in vivo by an oxidative pathway (cytochrome P-450) and conjugation pathway (glutathione S-transferase). The metabolites play an important role in its toxicity. Exposure to the higher concentrations in air causes burning of the eyes and upper airways irritation. Following central nervous system stimulation causing a deliriant picture, depression may develop resulting in unconsciousness or coma. Further vomiting can be observed. Hepatic and renal damage may also develop.
Inhaling large amounts of 1,2-dibromoethane severely irritates the mucous membranes, eyes and skin and respiratory system. People who inhale it become lightheaded, confused or sleepy. Rarely is anyone exposed to a fatal level since large amounts have a strong, sickening odor. The effects of breathing 1,2-Dibromoethane over a long time are not known. A study of workers found a high rate of deaths from tumors and diseases of the breathing system.
Long-term skin contact with 1,2-dibromoethane leads to redness, fluid build-up, blisters and ulcers. Swallowing or eating 1,2-dibromoethane can cause death from organ damage. The effects of drinking water containing low levels of 1,2-dibromoethane are not known. 1,2-dibromoethane affects sperm quality but does not lower birth rates. 1,2-dibromoethane is named as a substance that will probably cause cancer.
There is no direct antidote for 1,2-dibromoethane poisoning. A doctor will treat the symptoms based on the type of exposure. In some cases, physicians may use atropine.
References
[1] Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
[2] "Preparation and purification of 1,2-dibromoethane" (PDF). Synlett. 28: Supporting Information (Pages 49-51). 2017.
[3] Hamada Y., Mukai S. (1996). "Synthesis of ethano-Tröger's base using 1,2-dibromoethane". Tetrahedron Asymmetry. 7: 2671.
[4] Maynard, G. D. (2004). "1,2-Dibromoethane". In L. Paquette (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289 (inactive 2019-08-18).
[5] https://en.wikipedia.org/wiki/1,2-Dibromoethane
Related articles And Qustion
See also
Lastest Price from 1,2-Dibromoethane manufacturers

US $10.00-5.00/KG2025-06-27
- CAS:
- 106-93-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1.00/KG2024-10-11
- CAS:
- 106-93-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100000kg