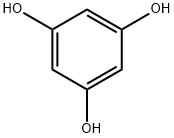
Phloroglucinol compounds of natural origin
General description
Phloroglucinol is a benzenetriol with hydroxy groups at position 1, 3 and 5. It has a role as an algal metabolite. Phloroglucinol is a natural product found in Artemisia dolosa, Koenigia coriaria, and other organisms with data available. Phloroglucinols include about 700 naturally occurring compounds with various biological activities. The enormous structural scope of this class ranges from 1,3,5-trihydroxy-benzene to the complex phlorotannins with 31 phloroglucinol moieties.Several syntheses of phloroglucinol have been reported, patented and commercialized; in particular. Phloroglucinols and their derivatives are an important class of secondary metabolites widely distributed in plants, marine organisms, and microorganisms. As one of the most important sources of natural phloroglucinols, Myrtaceous phloroglucinols discovered from the plants of family Myrtaceae are characterized by a polymethylated or formyl-substituted acylphloroglucinol moiety incorporating various kinds of biogenetic precursors via different coupling patterns. Such incorporations not only resulted in fascinating architectures and ring systems but also provided broader bioactivities than their parent compounds. On account of their intriguing chemical scaffolds and the biological significances, Myrtaceous phloroglucinols have attracted extensive attention from both chemical and biological communities in recent years. In just the past year, over 100 new natural phloroglucinol derivatives with various skeletons have been reported from family Myrtaceae[1,2].
Application and Pharmacology
The parent molecule phloroglucinol itself has been isolated from natural sources and is used in medicine, cosmetics, pesticides, paints, cements and dyeing.
1.Phloroglucinol is a non-atropine and non-papaverine pure smooth muscle antispasmodic drug. It has been investigated as a treatment for ureter, gastrointestinal tract and biliary tract diseases with its property of smooth muscle relaxation. Phloroglucinol has been used clinically to speed up labour. This study mainly assessed the clinical efficacy of phloroglucinol in improving the cervical conditions and promoting the progress of labour during natural obstetric delivery.The duration of first and second stages of labour is significantly reduced by phloroglucinol. The application of phloroglucinol is effective for accelerate the labour process and reduce the risk of complications, which is beneficial to maternal and fetal outcomes. However, the evidence is still insufficient. Large randomized controlled trials are needed to assess the effect of phloroglucinol on prolonged labour. The efficacy of the drug on post-partum hemorrhage, cervical dilatation, and pregnancy complications also requires further evaluation[3].
2.Phloroglucinol (1,3,5-benzenetriol; Flospan1) is a phenol derivative with anti-spasmodic properties. It suppresses spasms by normalizing smooth muscle movement, which is excessively stimulated by acetylcholine. As phloroglucinol selectively inhibits smooth muscle without anti-cholinergic action, it appears to be safe as a smooth muscle relaxant in patients with glaucoma and enlarged prostate. Furthermore, oral phloroglucinol is a transparent liquid that does not interfere with the endoscopic field of view and may therefore be suitable as a pre-treatment agent. A recent study on oral phloroglucinol as a premedication fordiagnostic EGD showed promising results, but that study lacked a placebo control. Therefore, we aimed to evaluate the effectiveness of oral phloroglucinol as a premedication for non-sedative EGD in a randomized, double-blinded, placebo-controlled trial. Phloroglucinol has been used in trials studying the diagnostic of Colonoscopy.Overall, 71 phloroglucinol-treated and 71 placebo-treated participants (n = 142 total) were included. The phloroglucinol group showed significantly higher proportions of participants with complete gastric peristalsis suppression than the placebo group (22.5% vs. 9.9%, P =0.040). The ease of intragastric observation was significantly better in the phloroglucinol group than in the placebo group at Periods A (P<0.001) and B (P = 0.005). Patients in both groups had comparable adverse events and showed willingness to take the premedication at their next examination. Oral phloroglucinol significantly suppressed gastrointestinal peristalsis during unsedated EGD compared with placebo[4].
3.Polycyclic polyprenylated acyl phloroglucinols have been a focus of numerous synthetic efforts. Their interesting biological activities (such as antidepressant, antianxiety, anti-Alzheimer, antiseptic, bactericidal and anti-inflammatory activities) mean that they will continue to attract the attention of synthetic chemists. Only one synthesis of macrocarpal C has been reported. Due to their anti-HIV activity and activity against periodontopathic bacteria, the synthesis of other macrocarpals should continue to be of considerable interest. Six different syntheses have been reported for the antimalarial robustadials, with the latest in 2006 from our laboratory. Few syntheses of higher phloroglucinols have been attempted, in part because their biological activities have not been well-explored. Considering the range of biological activities shown by various phloroglucinol molecules, we expect significant attention will be paid to syntheses of this group in the future.
Synthesis
Acyl phloroglucinols comprise the largest group of naturally occurring phloroglucinol compounds, and more than 100 simple acylated phlorolgucinols have been reported. These include phloroglucinol or its mono-, di- and tri-ethers acylated to varying degrees with linear or branched chains.
Figure 1 Overview of syntheses of phloroglucinol compounds from the parent molecule
The syntheses of variety of natural phloroglucinols. Synthesis of simple acyl phloroglucinols is unexceptional, but some of the compounds such as knipholone still present challenges to synthetic chemists because of chirality arising from their hindered biphenyl structure[2].
References
1.Su J., Wang S. & Cheng W. et al., "Phloroglucinol Derivatives with Unusual Skeletons fromCleistocalyx operculatus and Theirin Vitro Antiviral Activity," The Journal of Organic Chemistry, Vol.83, No.15(2018), pp.8522-8532.
2.Pal Singh I. & Bharate S. B., "Phloroglucinol compounds of natural origin," Natural Product Reports, Vol.23, No.4(2006), p.558.
3.Wu F., Chen Y. & Zheng C., "Efficacy of phloroglucinol for acceleration of labour: a systematic review and meta?analysis," Archives of Gynecology and Obstetrics, Vol.304, No.2(2021), pp.421-428.
4.Jung H., Kim H. J. & Choi E. S. et al., "Effectiveness of oral phloroglucinol as a premedication for unsedated esophagogastroduodenoscopy: A prospective, double-blinded, placebo-controlled, randomized trial," PLOS ONE, Vol.16, No.8(2021), p.e255016.
You may like
See also
Lastest Price from Phloroglucinol manufacturers
US $10.00/KG2025-04-21
- CAS:
- 108-73-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-04-15
- CAS:
- 108-73-6
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons