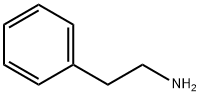
Application and Pharmacology of 2-phenylethanaminium
General description
Phenethylamine is a natural product found in Vitis vinifera, Trypanosoma brucei, and other organisms with data available. 2-phenylethylamine is a phenylethylamine having the phenyl substituent at the 2-position. It has a role as a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a phenylethylamine, an aralkylamine and an alkaloid. It is a conjugate base of a 2-phenylethanaminium.
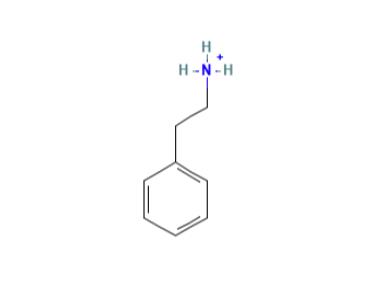
Figure1 The chemical structure of 2-phenylethylamine
Application and Pharmacology
1.2-Phenylethylamine is an important pharmaceutical and dye intermediate. In medicine, it is mainly used to synthesize the following drugs: excitatory drugs such as plant alkaloids ephedrine and cathinone, and synthetic drugs d-phenylisopropylamine and methylphenidate.
Hallucinogens such as plant alkaloid trimethoxyphenethylamine and synthetic drug 2C-B.
2.The phenethylamine hallucinogens are simple 2-phe-nylethylamine derivatives of the general formula I, the majority of which are substituted with methoxy and alkyl or halogen groups. Where is a methyl group, the com-pounds have been referred to as hallucinogenic “amphetamines.” Although not strictly correct, this nomenclature is used throughout this discussion. Although these drugs are not widely abused at present, several were prevalent on the illicit drug market during the 1960’s. The more common ones were 1-(2,5-dimethoxy- 4-methylphenyl)-2-aminopropane (“STP,” 11) (l), 1- (3,4-methylenedioxyphenyl)-2-aminopropane (111) (2), and 1 - (4-methoxyphenyl) -2-aminopropane (IV) (3). The latter two were responsible for several deaths. Their popularity was due to their relatively high potency, coupled with the economic attractiveness of starting materials such as an-isaldehyde, piperonal, and methylhydroquinone. With the exception of lysergide (LSD), the “classical” hallucinogens psilocin or psilocybin and mescaline were seldom available (4). Thus, the amphetamines served as convenient substitutes.
Mechanism of action
Phenethylamines are a large family of chemical structures that are molecular variants of the core compounds, i.e., amphetamines, MDMA, etc. The phenethylamine skeleton (1) consists of an aromatic ring with a side chain of two carbons ending by an amine group, as shown in Fig. 1. Slight modifications in this structure, partially induced by natural products, can change the pharmacological activity. Phenethylamines may thus undergo two major changes. First, substitution of the alpha carbon by a methyl gives rise to amphetamine derivatives (2), which have an optimal structure for psychostimulant activity. The 2C and D series are obtained by substitutions of the benzene cycle at positions 2 and 5 by methoxy groups and at position 4 by a variable substituent, respectively on phenethylamine (3) or amphetamine (4). These series have tetrahydrobenzodifuranyl and benzodifuranyl analogues (5) called "FLY" (Corazza et al., 2011). Finally, the NBOMe series which groups N-benzyl derivatives of the 2C series (6) has appeared more recently on the drug market.
Synthesis
The traditional experimental teaching method of “preparation of α-phenylehtylamine” is improved. By introducing Water knockout trap to avoid the acetophenone was distilled from the reactor and using environmental-friendly solvents– ethyl acetate and light petroleum to extract the product and the intermediate product. The steam distillation was replace by solvent extraction which shorten the experimental time. An excellent experimental teaching effect has been obtained by applying the improved method into the undergraduate experiment teaching:1.Traditional synthesis:
Add 11.8 ml acetophenone, 20 g ammonium formate and several zeolites into a 100 ml distillation bottle. A thermometer inserted into the bottom of the bottle is installed on the upper mouth of the distillation head, and the side mouth is connected with a condensing pipe to form a simple distillation device. Heat the reaction mixture on the asbestos net with a low fire to 150 ~ 155 ℃, ammonium formate begins to melt and divide into two phases, and gradually becomes homogeneous. The reactants are boiling fiercely, and water and acetophenone are steamed out. At the same time, they produce bubbles and emit ammonia. Continue heating slowly until the temperature reaches 185 ℃, stop heating, usually about 1.5 h. Transfer the distillate into the separating funnel, separate the acetophenone layer, pour it back to the reaction bottle, continue heating for 1.5 h, and control the reaction temperature not to exceed 185 ℃. Cool the reactant to room temperature, transfer it into the separating funnel, wash it with 15ml water to remove ammonium formate and formamide, and separate N-formyl - α- Return the crude phenylethylamine to the original reaction bottle. The water layer is extracted twice with 10ml chloroform each time, and the combined extract is also poured back to the reaction bottle and the water layer is discarded. Add 12 ml of concentrated hydrochloric acid and several zeolites into the reaction bottle, evaporate all chloroform, and then continue to keep slightly boiling and reflux for 30 ~ 45 minutes to make N-formyl - α - Phenylethylamine hydrolysis. The reactants were cooled to room temperature. Extract 3 times with 5 ml chloroform each time, and pour the combined extract into the designated recovery container. Transfer the water layer into a 100ml three neck bottle. Cool the three necked bottle in an ice bath, slowly add 20 ml of water solution and shake it, and then steam distillation. Check the distillate with pH test paper until pH = 7, and collect about 65 ~ 80 ml of distillate. Extract the distillate containing free amine with 10 ml toluene three times at a time, combine the toluene extract, add granular sodium hydroxide and dry. Add the dried toluene solution into a 25 ml distillation flask in batches with a dropping funnel, evaporate the toluene first, and then use an air condensing tube to collect the fraction at 180 ~ 190 ℃, with a yield of 5 ~ 6 g[2].
2.Improving synthesis:
(1) Avoid secondary reactions. The device used in the first step reaction is simple distillation. At this time, acetophenone and water will be evaporated at the same time. At the same time, the ammonia and carbon dioxide generated by the reaction will react to produce ammonium carbonate, and the solid will be condensed in the condenser. When the distillation temperature reaches 185 ℃ (about one and a half hours), stop heating, pour the acetophenone evaporated from the reaction back into the reaction bottle and continue the reaction for 1.5 hours.
(2) Green solvent instead of chloroform and toluene. In the second step, after washing, the crude product is extracted with organic solvent chloroform. Then hydrolyze under acidic conditions and distill chloroform. After the reaction, chloroform is used to extract the aqueous phase.In the last step, toluene is used as extractant to extract the final product.
(3) Desiccant is often used to replace sodium hydroxide. In the third step of alkalization reaction, the final product is evaporated by steam distillation, the distillate is extracted with toluene, and then dried with sodium hydroxide as desiccant. Finally, the former fraction toluene is evaporated by atmospheric distillation, and then the product is evaporated by air condensing tube.
Safety
Over the last decade, use of 2-phenethylamines has become increasingly prevalent. This study aimed to describe typical aspects of 2-phenethylamine poisoning in order to better inform patient care. Methods. Phenethylamine poisoning cases reported to the Poison Control Center of Angers, France, from January, 2007 to December, 2013 were examined. Clinical findings were examined in 105 patients, including phenethylamine used, symptoms and final outcome. Patients were predominantly male (80%), with mean age 26 ± 8 years. Results. MDMA (38%), amphetamine (18%) and methamphetamine (14%) were the most commonly reported. Synthetic cathinones (10%) and the 2C series (7%) were also found. Substances most commonly associated with phenethylamine poisoning were cannabis (27%), ethanol (20%) and cocaine (9%). The most frequently reported symptoms included anxiety and hallucinations (49%), mydriasis and headache (41%), tachycardia (40%) and hypertension (15%). Complications such as seizures (7%), cardiac arrest (5%), toxic myocarditis (1%) and hemorrhagic stroke (1%) were also observed. Of the cases, the Poison Severity Score was: null or low, 66%, moderate, 21%, severe or fatal, 13%. Of the patients, 77% received hospital care and 12.4% were admitted to an intensive care unit. Analytical confirmations were obtained for all severe cases. While 93% of patients recovered, there were 5 deaths and 2 patients presented with neurological sequelae. Conclusions. Phenethylamine poisonings may be severe in young and healthy individuals. Physicians, toxicologists and analysts should be aware of new 2-phenethylamine consumption trends in order to inform management of patient care and to contribute to a more responsive drug policy[3].
References
1.Borah A., Paul R. & Mazumder M. K. et al., "Contribution of β-phenethylamine, a component of chocolate and wine, to dopaminergic neurodegeneration: implications for the pathogenesis of Parkinson’s disease," Neuroscience Bulletin, Vol.29, No.5(2013), pp.655-660.
2.Ma Peng, Shi Zhifang, Zhang ronghua, etc.: green and efficient α- Synthesis of phenylethylamine - improvement of undergraduate teaching experiment, Guangdong chemical industry, 2013, No. 24, pp. 152-153.
3.Le Roux G., Bruneau C. & Lelievre B. et al., "Recreational phenethylamine poisonings reported to a French poison control center," Drug Alcohol Depend, Vol.154(2015), pp.46-53.