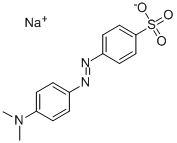
The synthesis of methyl orange
Introduction
Methyl orange is an organic substance with the chemical formula of C14H14N3SO3Na, which is often used as an acid-base indicator[1]. Methyl orange itself is weakly alkaline. The discoloration range of methyl orange is red when pH ≤ 3.1, orange when pH 3.1 ~ 4.4, and yellow when pH ≥ 4.4.

Picture 1 Methyl orange powders
Synthesis of methyl orange
1. Preparation of diazonium salt (diazotization reaction)[2]. Put p-aminobenzenesulfonic acid and 10ml of 5% sodium hydroxide solution into the beaker and dissolve it with warm heat. Take another small test tube and dissolve 0.8g sodium nitrite in 6ml water. Then pour the sodium nitrite solution into the cooled p-Aminobenzene Sulfonic Acid Solution and cool it to below 5 ° C with ice salt bath. Dilute 3ml of concentrated sulfuric acid (or concentrated hydrochloric acid) with 10ml of water, slowly add it to the above cooling mixture under continuous stirring, keep the temperature below 5 ° C, and then check it with starch potassium iodide test paper to show blue. In addition, if it does not show blue, add sodium nitrite solution.
2. Coupling reaction. Mix 1.3ml n, N-dimethylaniline and 1ml glacial acetic acid in a test tube and slowly add it to the diazonium salt solution. After addition, continue stirring for 10min, and then slowly add 5% sodium hydroxide solution (about 25ml) until the reactant turns orange. The crude methyl orange precipitated in fine particles. The reactant was heated on a boiling water bath for 5 minutes, cooled to room temperature, and then cooled in an ice water bath to completely precipitate the methyl orange crystal. Collect crystals by suction filtration, wash with a small amount of water, ethanol and ether in turn, and press dry. Dissolve a little methyl orange in water, add a few drops of dilute hydrochloric acid, and then neutralize it with dilute sodium hydroxide solution to observe the color change.
3. It is obtained by the reaction of p-Aminobenzene Sulfonic Acid with N, N-dimethylaniline after diazotization. Dissolve p-aminosulfonic acid in 2.5% sodium carbonate solution, add sodium nitrite, after complete dissolution, add crushed ice and hydrochloric acid for diazotization reaction, and precipitate diazonium salt crystals. Add the mixture of N, N-dimethylaniline and glacial acetic acid to the diazonium salt solution, stir for 10min, and then slowly add 10% sodium hydroxide solution to the alkaline solution. Cooling crystallization, filtration, washing with saturated brine, drying, recrystallization with water to obtain methyl orange.
4. Add 2kg p-aminobenzenesulfonic acid into the enamel container containing 6L 2mol / L sodium hydroxide and heat it to completely dissolve it (the solution is alkaline to litmus test paper). Then add 800g sodium nitrite, stir to dissolve, and cool to 5 ℃ with ice. The obtained solution was slowly added to the mixture of 5L 2mol / L hydrochloric acid and 10kg crushed ice under continuous stirring, cooled and stood for 15min. Determine the end point of diazotization reaction with starch potassium iodide test paper (the test paper turns blue). The prepared solution is added to the previously prepared cold solution of 1200gn, n-xylene amine and 10L 1mol / L glacial acetic acid under stirring. After mixing evenly, stand for 10min, slowly add 20% sodium hydroxide solution to obvious alkalinity, and precipitate yellow scaly methyl orange crystals. After heating the crude product until it is completely dissolved, add 2kg of chemically pure table salt, heat and stir until it is dissolved, cool and crystallize with water, filter by suction, and then dissolve it with a small amount of hot distilled water (1g of crystal is dissolved every 20ml of hot water), filter while it is hot, cool and crystallize the filtrate, suck it dry, and then wash it with a small amount of ethanol and ether successively, and dry it to obtain the finished product.
Reference
1 Fang Shijie, Xu Mingxia, Huang Weiyou, etc Photocatalytic degradation of methyl orange by nano-TiO2. Journal of silicate, 2001
2 Wang Yizhong, Fu Yan, Tang Hongxiao Study on heterogeneous photocatalytic degradation of methyl orange solution. Environmental science, 1998
Related articles And Qustion
See also
Lastest Price from Methyl Orange manufacturers
US $0.00/kg2025-10-31
- CAS:
- 547-58-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $25.00/ASSAYS2025-04-21
- CAS:
- 547-58-0
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt