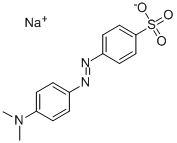
Methyl Orange: Applications in Biodiesel Production and Removal from Wastewater
General Description
In addition to being a dye, methyl orange can also be adsorbed on biochar and used as a catalyst to promote biodiesel production. However, wastewater containing methyl orange needs to be treated before it can be discharged. In wastewater treatment, Ugi multicomponent reaction-functionalized UiO-66-NS effectively removes methyl orange, boasting high adsorption capacity and stability across varying pH levels.

Figure 1. Methyl Orange
Applications in Biodiesel Production
Biodiesel production from non-edible oils utilizing a highly efficient eco-friendly catalyst is a crucial necessity for replacing fossil fuels. In the present work, biochar has been applied for both energy and environmental purposes. The biochar was made by slow pyrolysis from a variety of biomass, primarily cassava peel, irul wood sawdust, and coconut shell. All biochars were used as adsorbents to remove an anionic dye (methyl orange) by conducting batch adsorption studies.
The biochar made from cassava peels showed the highest dye adsorption, and it was characterized using elements analysis (CHNS), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), scanning electron microscopy (SEM), surface area analyzer (BET), total acid density, and sulfonic acid group density to successfully confirm the presence of weak (-OH) and strong (-COOH, -SO3H) acidic groups. Furthermore, for microwave-assisted biodiesel production from Millettia pinnata seed oil, the dye adsorbed biochar made from cassava peel was utilized as a Brønsted acid catalyst. The catalyst having a surface area of 4.89 m2/g, an average pore width of 108.77 nm, a total acid density of 3.2 mmol/g, and a sulfonic acid group density of 1.9 mmol/g exhibits distinctive mesoporous properties that contribute to a biodiesel yield of 91.25%. After being used in multiple cycles, it maintains a yield of over 75%, highlighting its durability and potential for reducing biodiesel production costs.1
Removal from Wastewater
The electrochemical method for treatment of organic waste-water has attracted a great deal of attention recently, mainly because of its ease of control, amenability to automation, high efficiency and environmental compatibility. Various electrochemical processes have been developed, among which electrochemical oxidation methods such as anodic oxidation or the oxidation by electrogenerated oxidizing agents might be the earliest and most extensively studied.
Electrochemical oxidation of methyl orange wastewater was studied using Ti/IrO2/RuO 2 anode and a self-made Pd/C O2-fed cathode in the divided cell with a terylene diaphragm. The result indicated that the appropriate rate of feeding air improved the methyl orange removal efficiency. The discoloration efficiency of methyl orange in the divided cell increased with increasing current density. The initial pH value had some effect on the discoloration of methyl orange, which became not obvious when the pH ranged from 2 to 10. However, the average removal efficiency of methyl orange wastewater in terms of total organic carbon (TOC) can reach 89.3%. The methyl orange structure had changed in the electrolytic process, and the characteristic absorption peak of methyl orange was about 470 nm. With the extension of electrolysis time, the concentration of methyl orange gradually reduced; wastewater discoloration rate increased gradually. The degradation of methyl orange was assumed to be cooperative oxidation by direct or indirect electrochemical oxidation at the anode and H2O2 , ·OH, O2- produced by oxygen reduction at the cathode in the divided cell. Therefore, the cooperative electrochemical oxidation of methyl orange wastewater in the anodic–cathodic compartment had better degradation effects.2
References:
[1] SUCHITH CHELLAPPAN. Methyl orange dye adsorbed biochar as a potential Br?nsted acid catalyst for microwave-assisted biodiesel production.[J]. Environmental Science and Pollution Research, 2023. DOI:10.1007/s11356-023-28269-3.[2] L PANG Z Y B H Wang. Treatment of methyl orange dye wastewater by cooperative electrochemical oxidation in anodic-cathodic compartment.[J]. Water Science and Technology, 2013, 67 3. DOI:10.2166/wst.2012.589.
You may like
Related articles And Qustion
See also
Lastest Price from Methyl Orange manufacturers
US $0.00/kg2025-10-31
- CAS:
- 547-58-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $25.00/ASSAYS2025-04-21
- CAS:
- 547-58-0
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt