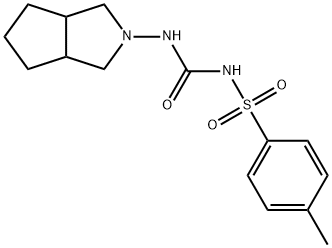
Pharmacokinetic study of the combination of Gliclazide
Gliclazide With Pedicellata
A study evaluated possible interaction between Ayurvedic anti-urolithiac agent hydroalcoholic extract of Didymocarpus pedicellata (HADP) leaves and gliclazide. Dose optimization performed by measuring serum glucose levels after 200 and 400 mg/kg HADP administration to normal rats. Pharmacokinetic interaction study in normal rats performed by administration of gliclazide alone and combination with HADP (400 mg/kg). Diabetes was induced by administration of streptozotocin (55 mg/kg) and animals were treated with gliclazide, HADP and combination for 28 days. Pharmacokinetic and dynamic interaction were assessed after single (day 1) and repeated dose (day 28) co-administration by determination of serum gliclazide and glucose levels respectively.

Gliclazide showed biphasic concentration time data and glucose reduction with maximum reduction at 2 and 8h post administration. HADP showed dose proportionate hypoglycemic effect in normal rats, hence 400 mg/kg was used for further studies. There was significantly higher decrease in percentage reduction of glucose levels in co-administration group as compared to gliclazide only group in normal, diabetic rats after single and repeated administration. Reduction was higher in repeated administration as compared to single. There was a non-significant increase in pharmacokinetic parameters in normal and diabetic rats after single HADP administration. Repeated HADP administration in diabetic rats caused significant increase in all pharmacokinetic parameters of gliclazide. On day 28 biochemical parameters are estimated to evaluate effect on oral administration of HADP with gliclazide for 28 days to diabetic animals. The results are shown a significant improvement in dyslipidemia, triglyceride levels and liver functional parameters such as SGOT, SGPT, ALP, and total protein in HADP combination as compared to the vehicle control group.
Combination of gliclazide and HADP showed a significant pharmacodynamic and pharmacokinetic interaction with gliclazide. Hence precautions has to be observed in co-administration of gliclazide with HADP and dosage adjustments of gliclazide might be required in a clinical setting to avoid sever hypoglycemia.
Gliclazide with Pravastatin
Diabetic hyperlipidemia is associated with increased lipoproteins in the blood hence, the introduction of a lipid lowering drugs aids in controlling the same. This comedication may lead to drug–drug interactions between anti-diabetic and anti-hyperlipidemic drugs. The studies conducted on rats dosed with gliclazide, pravastatin individually and in combination. Statistical comparisons of plasma concentration – response study in groups with gliclazide alone, pravastatin alone and in combination was carried out. The response study among concentrations and time were calculated employing student's paired T-Test.
The results indicate that gliclazide is completely absorbed with peak plasma concentration of 6.82±0.12 ng/ml and 7.82±0.12 ng/ml when administrated alone and 7.22±0.12 ng/ml and 7.22±0.12 ng/ml when administrated in combination in diabetic rats on first day (day 1) and eighth day (day8) respectively. Similarly peak plasma concentration of pravastatin are 3.92±0.03 ng/ml and 4.80±0.04 ng/ml when administrated alone and 3.683±0.02 ng/ml and 4.657±0.04 ng/ml when administrated in combination in diabetic rats on first day (day 1) and eighth day (day 8) respectively. There was no statistically noteworthy variation observed in peak plasma concentration (P>0.05). Similarly no variations observed in values of tmax, AUC and T1/2. The fasting serum glucose concentrations in normal and STZ-induced diabetic group on first day (day 1) and eighth day (day 8) were analyzed.
The reduction of blood glucose levels at different time intervals on administration of gliclazide and pravastatin alone and in combination analyzed and results indicate no significant change in pharmacodynamic parameters. Hence the results conclude that combinational therapy of gliclazide and pravastatin were found safe and highly potential in treating hyperlipidemia patients.
Gliclazide With Omeprazole
The antidiabetic drug gliclazide is partly metabolized by CYP2C19, the main enzyme involved in omeprazole metabolism. Developed PBPK models were verified using in vivo pharmacokinetic profiles obtained from a clinical trial on omeprazole-gliclazide interaction in healthy volunteers, CYP2C19 normal/rapid/ultrarapid metabolizers (NM/RM/UM). In addition, the association of omeprazole cotreatment with gliclazide-induced hypoglycemia was explored in 267 patients with type 2 diabetes (T2D) from the GoDARTS cohort, Scotland. The PBPK simulations predicted 1.4-1.6-fold higher gliclazide area under the curve (AUC) after 5-day treatment with 20 mg omeprazole in all CYP2C19 phenotype groups except in poor metabolizers.
The predicted gliclazide AUC increased 2.1 and 2.5-fold in intermediate metabolizers, and 2.6- and 3.8-fold in NM/RM/UM group, after simulated 20-day dosing with 40 mg omeprazole once and twice daily, respectively. The predicted results were corroborated by findings in patients with T2D which demonstrated 3.3-fold higher odds of severe gliclazide-induced hypoglycemia in NM/RM/UM patients concomitantly treated with omeprazole. Our results indicate that omeprazole may increase exposure to gliclazide and thus increase the risk of gliclazide-associated hypoglycemia in the majority of patients.
References:
[1] SATYANARAYANA CHINTHANIPPULA; Bimalendu C. Pharmacodynamic And Pharmacokinetic Interaction Of Didymocarpus Pedicellata With Gliclazide In Normal And Diabetic Rats[J]. Journal of Pharmaceutical Negative Results, 2023. DOI:10.47750/pnr.2023.14.s02.195.
[2] T. HASEEB; T. R. Pharmacokinetic and Pharmacodynamic Interactions Between Concomitantly used Gliclazide with Pravastatin[J]. International Journal of Pharmaceutical Sciences and Drug Research, 2020. DOI:10.25004/ijpsdr.2020.120612.
[3] TANJA DUJIC. Interaction between Omeprazole and Gliclazide in Relation to CYP2C19 Phenotype.[J]. Journal of Personalized Medicine, 2021. DOI:10.3390/jpm11050367.
You may like
Related articles And Qustion
Lastest Price from Gliclazide manufacturers

US $0.00-0.00/kg2025-09-11
- CAS:
- 21187-98-4
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 21187-98-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt