Gliclazide: Pharmacodynamic Properties, Pharmacokinetic Properties and Therapeutic Efficacy
General Description
Gliclazide is a medication used to reduce blood glucose levels in non-insulin dependent diabetes mellitus patients. It corrects defective insulin secretion and peripheral insulin resistance. The drug increases insulin secretion from pancreatic Jj-cells and improves their sensitivity to glucose. Gliclazide also has extrapancreatic effects, such as decreasing hepatic glucose production and increasing glucose clearance. It may improve haemobiological activity by reducing platelet adhesion and increasing tissue plasminogen activator levels. However, its effect on plasma lipids needs further investigation. Gliclazide is well-absorbed orally and achieves steady-state concentrations within 2 days. It has a low volume of distribution, extensive protein binding, and undergoes metabolism before excretion. Therapeutically, gliclazide effectively controls blood glucose levels, reduces glycosylated hemoglobin, and may delay the progression of diabetic retinopathy. Its impact on diabetic nephropathy and body weight requires more study.

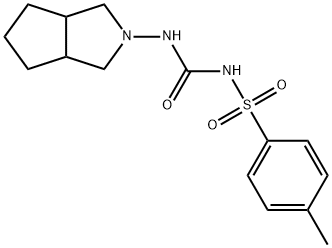
Figure 1. Gliclazide
Pharmacodynamic Properties
Gliclazide is a medication used to reduce blood glucose levels in non-insulin dependent diabetes mellitus patients by correcting both defective insulin secretion and peripheral insulin resistance. The drug increases unstimulated and stimulated insulin secretion from pancreatic Jj-cells, leading to an increase in intracellular calcium and induction of insulin release. Gliclazide also increases the sensitivity of Jj-cells to glucose; it may have extrapancreatic effects which restore peripheral insulin sensitivity, such as decreasing hepatic glucose production and increasing glucose clearance and skeletal muscle glycogen synthase activity. These effects do not appear to be mediated by an effect on insulin receptor number, affinity or function. Gliclazide also shows evidence of improving defective haemobiological activity in NIDDM patients by reducing platelet adhesion and increasing levels of tissue plasminogen activator, and reducing levels of free radicals. These properties appear to be specific actions of the drug on platelets and blood vessel walls, rather than an indirect effect of improved glycaemic control. However, data regarding its effect on plasma lipids are inconsistent, with reports of decreases or no change in plasma cholesterol and triglyceride levels after repeated administration. Further studies are needed to substantiate these findings. 1
Pharmacokinetic Properties
Gliclazide exhibits specific pharmacokinetic properties. When administered orally, its absorption is similar in both patients and healthy volunteers. However, there may be intersubject variation in the time to reach peak plasma concentrations (tmax), as well as age-related differences in peak plasma concentrations (Cmax) and tmax. Single-dose oral administration of gliclazide in doses ranging from 40 to 120mg results in a Cmax of 2.2 to 8 mg/L within 2 to 8 hours. With repeated administration, Cmax and tmax increase, but drug accumulation does not occur. Steady-state concentrations are achieved within 2 days of administering gliclazide in the same dose range. Taking gliclazide with food can reduce Cmax and delay tmax. The volume of distribution of gliclazide is low (13 to 24L) in both healthy volunteers and patients, which can be attributed in part to its extensive protein binding (85 to 97%). The elimination half-life (t'h) of gliclazide ranges from 8.1 to 20.5 hours after single or repeated oral administration. There may be age- and gender-related differences in t'h. The plasma clearance of gliclazide is approximately 0.78 L/h (13 ml/min). Gliclazide undergoes extensive metabolism, resulting in the formation of seven metabolites that are predominantly excreted in the urine, with the carboxylic acid derivative being the most abundant. About 60 to 70% of the dose is excreted in the urine and 10 to 20% in the feces. Although very little unchanged gliclazide is recovered in the urine, it represents over 90% of all drug-related material in the plasma. 2
Therapeutic Efficacy
Gliclazide has demonstrated significant therapeutic efficacy in controlling blood glucose levels among patients with non-insulin-dependent diabetes mellitus. Studies have shown that gliclazide effectively reduces fasting glucose levels by 12 to 62.1% and postprandial glucose levels by 18 to 26.7%, as well as the increase in plasma glucose induced by the glucose tolerance test. These effects are observed in both newly diagnosed and previously treated patients, indicating its broad applicability. Additionally, gliclazide leads to a reduction in glycosylated hemoglobin levels, indicating good glycemic control. When used in combination with insulin therapy, gliclazide allows for a reduction in insulin dosage, potentially reducing macroangiopathic complications of hyperinsulinemia. Furthermore, limited data suggest that gliclazide exhibits a low rate of secondary failure. Comparative trials have indicated that gliclazide is equivalent to other oral hypoglycemic agents in terms of glycemic control, while smaller studies suggest that long-term administration of gliclazide may delay the progression of diabetic retinopathy more effectively than other sulfonylureas or diet alone. However, these findings require further investigation for confirmation. Preliminary evidence suggests that gliclazide has minimal impact on diabetic nephropathy and body weight in both obese and non-obese patients. 1,2
Reference
1. Palmer KJ, Brogden RN. Gliclazide. An update of its pharmacological properties and therapeutic efficacy in non-insulin-dependent diabetes mellitus. Drugs. 1993;46(1):92-125.
2. Holmes B, Heel RC, Brogden RN, Speight TM, Avery GS. Gliclazide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in diabetes mellitus. Drugs. 1984;27(4):301-327.
Related articles And Qustion
See also
Lastest Price from Gliclazide manufacturers

US $0.00-0.00/kg2025-09-11
- CAS:
- 21187-98-4
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 21187-98-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt