Nilotinib: Pharmacokinetics, Tolerability and Dosage
General Description
Nilotinib, a medication used to treat imatinib-resistant Ph+ chronic-phase chronic myeloid leukaemia (CML) or Ph+ acute lymphoblastic leukaemia (ALL), has been studied pharmacokinetically in a phase I trial with 119 patients. The drug exhibits dose-dependent serum concentrations, surpassing the IC50 for BCR-ABL phosphorylation at doses of 50-1200 mg once daily and 400-600 mg twice daily. Steady-state levels increase up to 400 mg once daily, plateauing at higher doses due to likely gastrointestinal absorption saturation. Notably, 400 mg twice daily results in 35% higher drug exposure than 800 mg once daily, with no clinically relevant difference between 400 mg and 600 mg twice daily. Nilotinib's bioavailability rises by 82% when taken with a high-fat meal. Tolerability data from clinical trials indicate that the drug is generally well tolerated, with common adverse events including mild to moderate rashes, transient bilirubin level elevations, and gastrointestinal symptoms. The recommended dosage for imatinib-resistant or -intolerant Ph+ CML is 400 mg twice daily, with specific dietary instructions and precautions outlined in the US prescribing information. These findings provide crucial insights into the pharmacokinetics, tolerability, and dosing of nilotinib in the treatment of CML.

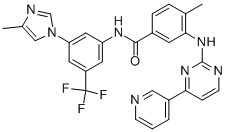
Figure 1. Nilotinib
Pharmacokinetics
Nilotinib, a medication used in treating imatinib-resistant Ph+ chronic-phase CML or Ph+ ALL, has been studied pharmacokinetically in a phase I trial with 119 patients. Oral administration at doses of 50-1200 mg once daily and 400-600 mg twice daily led to serum concentrations exceeding the IC50 for BCR-ABL phosphorylation, including most mutants. Steady-state levels increased up to 400 mg once daily, plateauing at higher doses due to likely gastrointestinal absorption saturation. Patients on 400 mg twice daily showed 35% higher drug exposure compared to 800 mg once daily, with no clinically relevant difference between 400 mg and 600 mg twice daily. At the recommended 400 mg twice daily, peak serum concentration reached 3.6 μmol/L after 3 hours, with a steady-state trough of 1.7 μmol/L. In healthy volunteers, nilotinib's bioavailability rose by 82% when taken with a high-fat meal. The drug is mainly bound to serum proteins (98%) with an unreported volume of distribution. Metabolism primarily occurs through oxidation and hydroxylation. Understanding these pharmacokinetic details is crucial for comprehending how nilotinib is absorbed, distributed, metabolized, and excreted in patients with imatinib-resistant Ph+ CML or Ph+ ALL. 1
Tolerability
Tolerability data for nilotinib, obtained from various clinical trials, provide valuable insights into its safety profile. The phase II trial focused on patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML) in chronic phase, involving 280 participants who received nilotinib at either 400 mg or 600 mg twice daily. The most common non-hematologic adverse events included mild to moderate rashes, transient elevations in bilirubin levels, and gastrointestinal symptoms such as nausea and pruritus. Additionally, hematologic adverse events like neutropenia and thrombocytopenia were observed, with dosage adjustments and supportive measures being necessary for some patients. Other laboratory abnormalities, such as elevated liver enzymes and lipase levels, were generally mild to moderate in severity. Despite these findings, the median duration of therapy and dose intensity indicate that nilotinib was generally well tolerated, with the planned dosage almost achieved. Furthermore, recent data suggest that indirect hyperbilirubinemia associated with nilotinib may result from a combination of drug inhibition and genetic polymorphism. Overall, these trials demonstrate the manageable tolerability profile of nilotinib in treating CML patients, providing important information for clinical decision-making. 2
Dosage
In patients with chronic- or accelerated-phase imatinib-resistant or -intolerant Ph+ CML, the recommended dosage of oral nilotinib is 400 mg twice daily. It is crucial that no food is consumed for at least 2 hours before or 1 hour after administration to optimize absorption. The US prescribing information highlights a boxed warning regarding the risk of sudden death and QTc interval prolongation associated with nilotinib use. Patients with hypokalaemia, hypomagnesaemia, or long QT syndrome should not use nilotinib. Prior to starting nilotinib, any existing hypokalaemia and hypomagnesaemia must be corrected, with regular monitoring thereafter. Healthcare providers should refer to local prescribing information for guidance on dosage adjustments, recommendations for special populations, contraindications, and precautions pertaining to nilotinib administration. 2
Reference
1. Deininger MW. Nilotinib. Clin Cancer Res. 2008;14(13):4027-4031.
2. Plosker GL, Robinson DM. Nilotinib. Drugs. 2008;68(4):449-461.
Related articles And Qustion
Lastest Price from Nilotinib manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 641571-10-0
- Min. Order:
- 10g
- Purity:
- 99%min
- Supply Ability:
- 5000g

US $10.00/KG2025-04-21
- CAS:
- 641571-10-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt