What is the difference between imatinib and nilotinib?
CML
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder characterized by the expansion of hematopoietic cells carrying the Philadelphia chromosome (Ph), resulting from a reciprocal translocation of the long arms of chromosomes 9 and 22. A novel fusion gene, BCR-ABL, is formed, which encodes a constitutively active protein tyrosine kinase[1].
Nilotinib
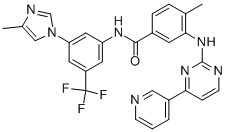
Nilotinib (Tasigna; N)-[3-[3-(1H-imidazolyl)propoxy] phenyl]-4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino] benzamide; Novartis Pharmaceuticals) is a tyrosine kinase inhibitor (TKI) approved for the treatment of adult patients with newly diagnosed Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) or imatinib-resistant or imatinib-intolerant Ph+ CML in CP or accelerated phase. Tyrosine kinases are proteins that act as chemical messengers and can stimulate cancer cells to grow.
It was first approved on October 29, 2007. On March 22, 2018, the Food and Drug Administration approved nilotinib (TASIGNA, Novartis Pharmaceuticals Corporation) for pediatric patients 1 year of age or older with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) or Ph+ CML-CP resistant or intolerant to prior tyrosine-kinase inhibitor (TKI) therapy.
Imatinib
Imatinib is a type of cancer growth blocker called a tyrosine kinase inhibitor (TKI). Tyrosine kinases are proteins that cells use to signal to each other to grow. They act as chemical messengers. There are a number of different tyrosine kinases, and blocking them stops cancer cells from growing.
Research
Based on understanding the molecular mechanism of imatinib activity, structural modifications led to the development of nilotinib. Like imatinib, nilotinib inhibits Bcr-Abl by binding to an inactive, DFG-out conformation of the ABL kinase domain, thus preventing the enzyme from adopting the catalytically active conformation and blocking the tyrosine phosphorylation of proteins involved in Bcr-Abl signal transduction. The improved binding of nilotinib results in greater potency and selectivity over the KIT and PDGF receptor kinases, but it has no activity against targets such as the Src-family of tyrosine kinases. In preclinical models, nilotinib was 30 times more potent than imatinib in imatinib-sensitive CML cell lines. It maintained activity in 32 of 33 imatinib-resistant BCR-ABL mutant cell lines, encouraging its development in CML. Results from a phase 1 dose-escalation study performed in patients with imatinib-resistant CML and Ph+ acute lymphoblastic leukemia (ALL) indicated that nilotinib produced significant hematologic and cytogenetic responses in all phases of CML. Side effects potentially related to nilotinib included grade 3 or 4 hematologic toxicity, with cytopenias in 6% to 20% of patients, as well as transient indirect hyperbilirubinemia and skin rash.
Adverse events with a suspected relationship to nilotinib are summarized below. These were generally mild to moderate in severity. The most commonly reported nonhematologic events considered possibly related to nilotinib and of any grade severity were rash (28%), nausea and pruritus (24% each), and headache and fatigue (19% each). Grade 3 or 4 toxicities were noted in 3% or less patients. Clinically notable adverse events reported with other Bcr-Abl inhibitors, such as pleural effusions, pericardial effusions, pulmonary edema, and left ventricular dysfunction, were rarely observed with nilotinib[2].
References
[1] A Hochhaus. “Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial.” Leukemia 30 5 (2016): 1044–1054.
[2] Hagop M Kantarjian. “Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance.” Blood 110 10 (2007): 3540–6.
Related articles And Qustion
See also
Lastest Price from Nilotinib manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 641571-10-0
- Min. Order:
- 10g
- Purity:
- 99%min
- Supply Ability:
- 5000g

US $10.00/KG2025-04-21
- CAS:
- 641571-10-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt