Methyl Benzoylformate: Photoinitiator Use & Thermal/Reduction Reactions
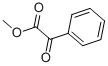
Methyl benzoylformate, also known as methyl phenylacetonate, photoinitiator MBF, etc., molecular formula C₉H₈O₃, CAS No. 15206-55-0. Its appearance is a light yellow transparent liquid, is an important raw material for organic synthesis, the core use is as a photoinitiator application. Its core use is as a light initiator. It needs to be stored in room temperature, dry and sealed environment, slightly irritating, need to wear protective equipment such as goggles, gloves, etc. The safety statement is S24/25, low toxicity, rabbit oral LD50 is more than 5000mg/kg.

Magnesium(II) ion-activated reduction of methyl benzoylformate
The nicotinamide adenine dinucleotide phosphate NAD(P)H/NAD(P)+ redox system is of major importance for several biological processes such as photosynthesis, glycolysis, fatty acid synthesis, citric acid cycle, and amino acid metabolism. Many organic and bioorganic chemists have been challenged to mimic this type of biochemical reaction, and since Ohno and co-workers. We present herein a detailed computational model to explain the enantioselective reduction of methyl benzoylformate with peptide-containing NADH mimetics under nonenzymatic conditions. The proposed model is based on the combination of two structural features: first, the chelation of NADH peptides with Mg2+ cation to form a seven-membered ring, and second, the application of the Freidinger’s lactam peptidomimetic. Enantioselective reduction of methyl benzoylformate with the NADH models was investigated in the presence of magnesium perchlorate in acetonitrile at room temperature, and the results obtained are summarized. A uniform stereoinduction tendency was observed in all instances affording the S-(+) enantiomer of methyl mandelate as the major reaction product.[1]
To study the reaction mechanism and to ascertain the origin of the stereoselectivity observed for the reduction of methyl benzoylformate with NADH β-lactam peptidomimetics, we selected first the model structure. The flexible benzylic moieties in compounds 5 and the bulky CH(SiMe3)2 were replaced by the less demanding and more rigid two methyl groups to shorten the computational time and limit the conformational energy. For the initial model, all structures were optimized by using the density functional B3LYP. Strongly rigidified β-lactam peptidomimetic NADH models have been used to investigate the ternary entities NADH/Mg2+/PhCOCO2Me taking part in the biomimetic reduction of methyl benzoylfomate. According to computational calculations at the B3LYP/6-31G* level, up to 10 stable ternary complexes can be formed by the accommodation of an s-cis-methyl benzoylformate ligand around a seven-membered chelation pseudoplane encompassing the Mg2+ cation, the β-lactam carbonyl, and the dihydronicotinamide carbonyl. Calculations have also allowed an unambiguous identification of the “productive” ternary complexes, as well as the corresponding transition states leading to the (S)- and (R)-methyl mandelates with activation energy differences about 3−4 kcal/mol.
Unimolecular gas phase thermal decomposition of the α-ketoester methyl benzoylformate
Among the many different reaction systems, pressure- and temperature-dependence kinetics of gas phase unimolecular reactions play important roles. The gas phase thermal decomposition of methyl benzoylformate (methyl oxophenylacetate) has been recognized as unimolecular elimination reaction of α-ketoesters. This reaction at 600 K proceeds via a five-membered cyclic transition state to yield benzaldehyde and α-acetolactone; and subsequently continues with decomposition of α-acetolactone into formaldehyde and carbon monoxide. In spite the existence of experimental and theoretical studies about the unimolecular elimination reaction of α-ketoesters, no evidences are reported about the pressure-dependence kinetics and molecular mechanism of thermal decomposition of methyl benzoylformate to date. To predict the change of rate constant with pressure of buffer gas and to explain the formation/breaking of chemical bonds, creation/annihilation of lone electron pairs, etc., along the reaction course, a theoretical examination is unavoidable. Actuated by such a goal, the theoretical study has been carried out in order to shed light mentioned unknown issues for unimolecular elimination reaction of methyl benzoylformate, through three-membered cyclic transition state, by means of statistical Rice-Ramsperger-Kassel-Marcus (RRKM) model at the experimentally employed temperature and bonding evolution theory (BET) analysis.[2]
A comprehensive study has been carried out on the kinetics and molecular mechanism of gas phase thermal decomposition of methyl benzoylformate by quantum chemical calculations. The reaction kinetics was investigated in the framework of RRKM theory over a wide range of pressures at the experimentally employed temperature 733 K. Decomposition of methyl benzoylformate is exergonic which high-pressure limit and transition pressure in the fall-off curve were found lower than atmospheric pressure, indicating the unimolecular rate coefficient under the atmospheric condition is in the high-pressure limit. Low-pressure limit rate coefficients were calculated by using different collisional efficiency values and was in the range of 10−13-10−12 cm3 molec−1 s−1.
References
[1]Aizpurua JM, Palomo C, Fratila RM, Ferrón P, Benito A, Gómez-Bengoa E, Miranda JI, Santos JI. Mechanistic insights on the magnesium(II) ion-activated reduction of methyl benzoylformate with chelated NADH peptide beta-lactam models. J Org Chem. 2009 Sep 4;74(17):6691-702. doi: 10.1021/jo901236d. PMID: 19642693.
[2]Keley V, Zahedi E. Understanding the kinetics and molecular mechanism of unimolecular gas phase thermal decomposition of the α-ketoester methyl benzoylformate using RRKM and BET theories. J Mol Graph Model. 2019 Mar;87:22-29. doi: 10.1016/j.jmgm.2018.11.004. Epub 2018 Nov 15. PMID: 30472634.
You may like
Related articles And Qustion
See also
Lastest Price from Methyl benzoylformate manufacturers
US $1.00/KG2025-04-21
- CAS:
- 15206-55-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $150.00/kg2025-04-21
- CAS:
- 15206-55-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg