EPA: Cardiovascular Benefits & Atrial Fibrillation Risk
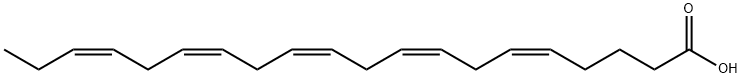
Timnodonic Acid is a polyunsaturated fatty acid, usually found in fish oils, that is used in many supplements. EPA can be used for lowering elevated triglycerides in those who are hyperglyceridemic. In addition, it may play a therapeutic role in patients with cystic fibrosis by reducing disease severity and may play a similar role in type 2 diabetics in slowing the progression of diabetic nephropathy. Timnodonic Acid is also used to associated treatment for these conditions: Dietary and Nutritional Therapies. The anti-inflammatory, antithrombotic and immunomodulatory actions of EPA is probably due to its role in eicosanoid physiology and biochemistry. Most eicosanoids are produced by the metabolism of omega-3 fatty acids, specifically, arachidonic acid. These eicosanoids, leukotriene B4 (LTB4) and thromboxane A2 (TXA2) stimulate leukocyte chemotaxis, platelet aggregation and vasoconstriction. They are thrombogenic and artherogenic. On the other hand, it is metabolized to leukotriene B5 (LTB5) and thromboxane A3 (TXA3), which are eicosanoids that promote vasodilation, inhibit platelet aggregation and leukocyte chemotaxis and are anti-artherogenic and anti-thrombotic. The triglyceride-lowering effect of EPA results from inhibition of lipogenesis and stimulation of fatty acid oxidation. Fatty acid oxidation of which occurs mainly in the mitochondria. EPA is a substrate for Prostaglandin-endoperoxide synthase 1 and 2. It also appears to affect the function and bind to the Carbohydrate responsive element binding protein (ChREBP) and to a fatty acid receptor (G-coupled receptor) known as GP40.

Emerging Pathways of Action of Eicosapentaenoic Acid (EPA)
Yet, lack of a clear understanding of the likely multifactorial mechanism of benefit of EPA has generated skepticism among some physicians, potentially leading to underuse of this clinically beneficial, cost-effective, and safe therapy. The REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) showed large relative and absolute risk reductions in ischemic events with icosapent ethyl (a highly purified, pharmaceutical grade ethyl ester of EPA), including a significant reduction in cardiovascular death in secondary and high-risk primary prevention patients with mildly to moderately elevated triglycerides. Interestingly, the benefit extended across the full range of baseline triglyceride values in the trial, including in the subgroup of patients who entered the trial with normal triglyceride levels. Similarly, the benefit of icosapent ethyl extended to those patients who either did or did not achieve triglyceride levels within the normal range during the trial. Mediation analyses suggested that the triglyceride reduction accounted for a minority of the drug’s benefit, with approximately two-thirds of the benefit caused by the large increase in serum EPA that occurred on treatment. However, that observation does not actually explain how the EPA itself exerts its cardioprotective benefits.[1]
Inflammation contributes centrally to the genesis of plaque rupture and ischemic events. Contemporary data support that the risk associated with arterial inflammation persists independently of low-density lipoprotein cholesterol and triglyceride levels. The data from Reilly et al suggest that EPA could help address this component of residual cardiovascular risk. Analyses from REDUCE-IT show consistent clinical benefits of EPA across baseline levels of low-density lipoprotein cholesterol (and triglycerides, as noted in the previous text). Anti-inflammatory effects mediated by the adaptive immune modulation beyond those assessed by the biomarkers of innate immunity previously reported in REDUCE-IT, such as the effects on T cells reported by Reilly et al, could contribute to these benefits. Carefully performed basic science experiments such as the current ones by Reilly et al should help convince physicians that data from REDUCE-IT and several other consistent clinical and imaging trials have a firm biological basis. Hopefully, this deeper understanding of the emerging pathways of action of EPA will translate into appropriate patients being identified and treated with icosapent ethyl in daily practice.
EPA: between Cardiovascular Benefits and the Risk of Atrial Fibrillation
Omega-3 polyunsaturated fatty acids (n-3PUFAs) are a family of fatty acids characterized by the closest double bond to the methyl end of the hydrocarbon chain to be on the third-to-last carbon. The most studied n-3PUFAs are represented by eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), which play a pivotal role in cellular biology regulating cell phospholipidic membrane’s fluidity. Particularly, the augmented risk of AF has been outlined over the years, even recently, with conflicting results. Definitive evidence on the causal role in AF of n-3PUFAs and their putative mechanisms is still lacking; in fact, it remains unclear whether this adverse event could be class- or molecule-specific or dose-dependent. To ensure an adequate evaluation of the risk/benefit ratio of n-3PUFAs treatment, an accurate analysis should be conducted. Our goal is, therefore, to investigate recent evidence for the cardiovascular benefit of EPA therapy and the risk of developing AF. Eventually, we propose a hypothetical flowchart as a base for subsequent studies aimed to formally define a clinical algorithm for safer and more effective prescription of EPA in patients.[2]
In recent years, scientific research has increasingly focused on the cardiovascular benefits of omega-3 polyunsaturated fatty acids (n-3 PUFAs) supplements. The most promising results emerged from the new trials on a high-dose eicosapentaenoic acid (EPA)-only approach, instead of the previously prescribed therapy with EPA + docosahexaenoic acid (DHA). The evidence of the reduction of cardiovascular events in patients at high cardiovascular risk with EPA is intriguing. However, physicians have expressed concern about the potential high risk of atrial fibrillation (AF) occurrence due to such an approach. This study aims to investigate the current evidence on the cardiovascular benefits of it and its association with atrial arrhythmogenesis. Current guidelines consider it (as IPE) treatment for selected patients but with no specific indication regarding AF risk evaluation. We propose a flowchart that could be a starting point for the future development of an algorithm to help clinicians to prescribe EPA safely and effectively, especially in patients at high risk of incipient AF.
References
[1]Bhatt DL, Libby P, Mason RP. Emerging Pathways of Action of Eicosapentaenoic Acid (EPA). JACC Basic Transl Sci. 2025 Mar;10(3):396-400. doi: 10.1016/j.jacbts.2024.10.010. PMID: 40139880; PMCID: PMC12013833.
[2]Egalini F, Rossi M, Massussi M, Gaggero G, Beccuti G, Benso A, Piepoli MF, Broglio F. Eicosapentaenoic Acid: between Cardiovascular Benefits and the Risk of Atrial Fibrillation. Endocr Metab Immune Disord Drug Targets. 2024;24(6):651-663. doi: 10.2174/0118715303280825231122153024. PMID: 38083891; PMCID: PMC11275313.
You may like
Lastest Price from EPA manufacturers

US $79.00-38.00/kg2025-04-21
- CAS:
- 10417-94-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

US $0.00-0.00/g2024-12-24
- CAS:
- 10417-94-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 20 tons