Methyl benzoylformate: properties, applications and safety
General Description
Methyl benzoylformate is a chemical compound with a molecular formula of C9H8O3, known for its moderate solubility in water and primarily soluble in organic solvents. It can undergo ester hydrolysis under certain conditions and exhibits photoinitiating properties when exposed to ultraviolet light. Methyl benzoylformate finds extensive use in various industries, including UV-curable coatings and inks, agriculture, and pharmaceuticals. However, it may cause an allergic skin reaction, and necessary precautions such as wearing protective clothing and avoiding inhalation or ingestion should be taken while handling this chemical compound.

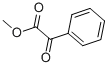
Figure 1. Methyl benzoylformate
Properties
Methyl benzoylformate is a chemical compound with several notable properties. Firstly, it has a molecular formula of C9H8O3 and a molecular weight of 164.16 g/mol. It appears as a colorless to pale yellow liquid with a sweet, fruity odor. Methyl benzoylformate is known for its moderate solubility in water. It is primarily soluble in organic solvents such as ethanol, ether, and acetone. In terms of reactivity, Methyl benzoylformate can undergo ester hydrolysis under certain conditions, leading to the formation of benzoylformic acid. It is sensitive to air, light, and moisture and should be stored in a cool, dry place to maintain its stability. Methyl benzoylformate exhibits photoinitiating properties when exposed to ultraviolet light, making it valuable in the field of UV-curable coatings and inks. It initiates the polymerization process, resulting in fast drying and curing of coatings and inks. Overall, Methyl benzoylformate possesses important properties such as solubility, volatility, reactivity, and photoinitiating capabilities, which contribute to its usefulness in various industrial applications. 1
Applications
Methyl benzoylformate is a versatile chemical compound with a wide range of applications. One of its main applications is in the field of UV-curable coatings and inks. Methyl benzoylformate acts as a photoinitiator, which means it initiates the polymerization process when exposed to ultraviolet light. This property makes it an essential component in UV-curable formulations, where it helps in the rapid drying and curing of coatings and inks. UV-curable coatings find applications in various industries, such as automotive, electronics, and packaging, due to their fast curing times and excellent durability. Another important application of methyl benzoylformate is as an intermediate in the synthesis of the herbicide Benzimidazolinone. Benzimidazolinone is widely used as a selective herbicide, primarily in the agricultural sector, to control the growth of unwanted weeds. Methyl benzoylformate plays a crucial role in the production of Benzimidazolinone, enabling the synthesis of this potent herbicide. Furthermore, methyl benzoylformate finds extensive use in organic synthesis. It serves as a versatile building block for the preparation of various compounds with diverse functionalities. Its chemical reactivity allows for the introduction of different functional groups, making it valuable for the synthesis of pharmaceuticals, agrochemicals, and other specialty chemicals. In summary, methyl benzoylformate is a valuable compound with multiple applications. Its role as a photoinitiator in UV-curable coatings and inks, its use as an intermediate in the production of herbicides, and its versatility in organic synthesis highlight its significance in various industries, including coatings, agriculture, and pharmaceuticals. 2
Safety
Methyl benzoylformate may cause an allergic skin reaction. Therefore, it is important to take necessary precautions while handling this chemical compound. To prevent any adverse effects, it is recommended to wear protective gloves, protective clothing, eye protection, face protection, and hearing protection when working with Methyl benzoylformate. Contaminated work clothing should not be taken out of the workplace. Avoid breathing in dust, fumes, gases, mists, vapors, or spray of this substance. In case of skin contact, remove contaminated clothing and wash the affected area before reuse. Seek medical assistance if skin irritation or rash occurs. If Methyl benzoylformate comes into contact with your eyes, flush them with water as a precautionary measure. Ingestion of the chemical should be avoided, and if swallowed, rinse the mouth with water. If this substance is inhaled, move the affected person to fresh air. In the event of a fire involving Methyl benzoylformate, use water spray, alcohol-resistant foam, dry chemical, or carbon dioxide as suitable extinguishing media. Firefighters should wear self-contained breathing apparatus if necessary. It is important to store Methyl benzoylformate in a cool place, tightly closed in a dry and well-ventilated area. Keep it in suitable, closed containers for disposal. 3
Reference
1. PubChem. Compound Summary: Methyl benzoylformate. National Library of Medicine, 2005, CID:84835.
2. Methyl Benzoylformate Derivative Norrish Type I Photoinitiators for Deep-Layer Photocuring under Near-UV or Visible LED. Macromolecules, 2021, 54, 8:3854-3864.
3. Chemical Safety Data Sheet: Methyl benzoylformate. ChemicalBook, 2023, CBnumber: CB9264481.
Related articles And Qustion
See also
Lastest Price from Methyl benzoylformate manufacturers
US $1.00/KG2025-04-21
- CAS:
- 15206-55-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $150.00/kg2025-04-21
- CAS:
- 15206-55-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg