Lithium Dihydrogen Phosphate: LLC Mesophase Behaviors and NCA Cathode Modification Effects
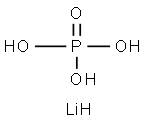
Lithium dihydrogen phosphate is a significant inorganic compound appearing as a white crystalline powder with the molecular formula LiH₂PO₄ and a molecular weight of 71.99. It exhibits distinct solubility characteristics, being readily soluble in water, slightly soluble in ethanol, and insoluble in ether. This property provides fundamental conditions for its subsequent processing and application. As a material of exceptional performance, lithium dihydrogen phosphate's foremost advantage lies in its outstanding physicochemical properties. It possesses high electrical conductivity, effectively facilitating charge transfer. Concurrently, it exhibits excellent thermal and chemical stability, resisting decomposition or chemical reactions when subjected to temperature fluctuations or contact with other substances. This renders it an ideal electrolyte material, playing a pivotal role in scenarios requiring stable charge conduction.

Lyotropic Liquid Crystalline Mesophases of Lithium Dihydrogen Phosphate
Lyotropic liquid crystalline (LLC) mesophases are important in the development gel-electrolytes. Salt-surfactant and acid-surfactant LLC mesophases have a high ionic-conductivity (0.1–20 mS/cm) and they have been employed as gel-electrolytes in a number of electrochemical devices, such as electrochromic displays, solar cells, acid batteries, optical modulators, lithium-ion batteries, super capacitorsetc. Water in the mesophase plays a critical role in determining the structural properties and ionic-conductivity of the LLC gel-electrolytes. Lithium dihydrogen phosphate is one of those salts that have very little or no water tolerance. Therefore, evaporation of hydration water from the Li−EO−X mesophase results a separation of salt and surfactant. However, a stable Li−EO−X mesophase will be very beneficial for both gel-electrolytes (as both lithium and proton conductors) and for the synthesis of mesoporous lithium metal phosphates (LMPs), which are important in lithium-ion batteries. We have shown that lithium nitrate-transition metal nitrate−phosphoric acid-surfactant mesophases can be used to synthesize mesoporous LMPs. The use of Lithium dihydrogen phosphate, as a lithium and phosphate source in the synthesis of mesoporous LMPs, can be beneficial as it eliminates some of the nitrates that require high temperatures to remove from the LMPs. Therefore, it is worth investigating the phase behaviors and stability issues of the Li−EO−X mesophases towards the above two goals.[1]
In summary, the Li−EO−X mesophase can be prepared with salt/surfactant mole ratios between 2 and 200, but the mesophases are not stable; at lower concentrations it leaches out surfactant, and at higher concentrations it leaches out both surfactant and salt crystals. The stability of the mesophase is improved by increasing amount of salt in the media. At high salt concentrations, enough water is kept in the mesophase for a longer duration; however the Lithium dihydrogen phosphate salt cannot indefinitely keep those water molecules in the mesophase, therefore it undergoes phase separation into salt and surfactant crystals over time. Once the LLC mesophase is formed with evaporation of excess water, the mesophase starts leaching out first surfactant to increase salt/surfactant mole ratio in the mesophase, then later salt crystals with further water-loss. However, the crystallization can be postponed by adjusting the humidity of the environment. High humidity not only hinders the crystallization but also dissolves the already crystallized salt species back into the mesophase. Once the H3PO4 amount exceeds the Lithium dihydrogen phosphate amount in the phase, the salt crystallization can be stopped. Evaporation of the hydration water may not only influence the stability of the phase but may also determine the mesostructure.
Enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2cathode by LiH2PO4
The exposed NCA particles are susceptible to corrosion by Lewis acid, resulting in the dissolution of transition metal ions from the lattice into the electrolyte or even shuttle to the negative electrode, hence the lattice structure undergoes continuous degradation. As the side reaction continues, the reversible capacity gradually decreases and the cycle stability deteriorates. In order to solve these problems, surface coating of NCA is a particularly effective strategy, which can block the direct contact between NCA and electrolyte, thereby reducing the side reactions. However, the traditional coating method rarely optimizes the residual alkali on the surface and the majority of the coating materials are Li-ion insulators with lower Li-ion diffusion coefficient, which hinders Li-ion transportation at the electrode interface and thus leads to the poor rate capability. Considering these circumstances, we develop a new coating material, Lithium dihydrogen phosphate, to modify the surface of NCA to enhance electrochemical performance, which has never been reported before. Notably, the Lithium dihydrogen phosphate can not only react with residual alkali on the surface to remove H2O and CO2, but also can form a coating layer with excellent ion conduction to enhance its surface ionic conductivity.[2]
The presence of residual alkali (LiOH and Li2CO3) on the surface will accelerate its reaction with HF from LiPF6, resulting in structural degradation and reduced safety. In this work, we develop a new coating material, Lithium dihydrogen phosphate, which can effectively optimize the residual alkali on the surface of NCA to remove H2O and CO2 and form a coating layer with excellent ion conductivity. Under this strategy, the coated sample NCA@0.02Li3PO4 (P2-NCA) provides a capacity of 147.8 mAh g−1 at a high rate of 5 C, which is higher than the original sample (126.5 mAh g−1). Impressively, the cycling stabilities of P2-NCA under 0.5 C significantly improved from 85.2% and 81.9% of pristine-NCA cathode to 96.1% and 90.5% at 25 °C and 55 °C, respectively. These satisfied findings indicate that this surface modification method provides a feasible strategy toward improving the performance and applicability of nickel-rich cathode materials.
References
[1]Topuzlu, Ezgi Y?lmaz et al. “Lyotropic Liquid Crystalline Mesophases of Lithium Dihydrogen Phosphate and 10-Lauryl Ether Stabilized with Water or Phosphoric Acid.” ChemPlusChem vol. 88,1 (2023): e202200447. doi:10.1002/cplu.202200447
[2]Cheng, Wendong et al. “Optimizing surface residual alkali and enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2cathode by LiH2PO4.” Nanotechnology vol. 33,4 10.1088/1361-6528/ac2f58. 3 Nov. 2021, doi:10.1088/1361-6528/ac2f58
You may like
Lastest Price from Lithium dihydrogen phosphate manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 13453-80-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $20.00-2.00/kg2025-03-07
- CAS:
- 13453-80-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons