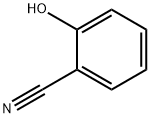
2-Cyanophenol: Conformational Interconversion & Matrix-Isolated Photochemistry
2-Cyanophenol is a phenolic compound that is used as an intermediate in the synthesis of pharmaceuticals and agrochemicals. It is also a disinfectant used for wastewater treatment and to remove salicylaldoxime from contaminated water.

The Conformational Interconversion of 2-Cyanophenol
2-Cyanophenol (2CP) is a phenol derivative substituted at the ortho position by a cyano group (−C≡N). In this molecule, two distinct and stable orientations are possible for the OH group, which are commonly called cis or trans, depending on the fact that whether the OH is pointing toward the substituent or away from it, respectively. Crystallographic, thermochemical, and spectroscopic studies have been performed on 2-Cyanophenol. Particularly relevant for its conformational characterization were the results obtained by laser-induced fluorescence excitation spectroscopy of jet-cooled molecules and microwave spectroscopy of the gaseous compound. In those studies, the low-energy conformer (cis) was spectroscopically characterized, whereas the trans conformer was not detected because of its low abundance in gas phase. Moreover, only the cis form was identified in a low-temperature argon (Ar) matrix when 2CP was generated by UV irradiation of 1,2-benzisoxazole. A commercial sample of 2CP (Sigma-Aldrich, 99% purity), gaseous nitrogen (Air Liquide, N50), and argon (Air Liquide, N60) were used in the experiments. To prepare a low-temperature solid matrix, a small amount of solid 2-Cyanophenol was placed inside a miniature glass recipient, which was connected to the vacuum chamber of a closed-cycle helium cryostat (APD Cryogenics, with a DE-202A expander).[1]
Conformational isomerism in 2-Cyanophenol was studied for the matrix-isolated compound, using IR spectroscopy supported by quantum-chemical calculations. This molecule has two planar conformers: cis and trans, the first being more stable because of the formation of a weak O–H···N≡C hydrogen bond-like interaction. Narrowband irradiation of 2CP isolated in solid N2 with NIR laser light tuned at the wavenumbers of the 2ν(OH) transitions of the conformers led to a bidirectional conversion between the two forms. To the best of our knowledge, observation of this type of processes in phenol-type compounds had only been reported for gallic acid. This also allowed, for the first time, the spectroscopic characterization of the high-energy trans conformer of 2-Cyanophenol. The effect of the spectrometer broad-band IR source was investigated by preparing different relative populations of the two 2CP conformers in the N2 matrix and subjecting it to diverse experimental conditions. The performed detailed analyses of the IR radiation and tunneling effects on the conformational isomerization reactions, together with the expected stabilization of the trans conformer in the N2 matrix through a specific interaction between the OH group and the N2 molecules, also allowed to explain the different cis:trans population ratios observed after isolating monomers of 2-Cyanophenol in the Ar and N2 matrixes.
2-Cyanophenol Isolated in Low-Temperature Ar Matrixes
The monomers of 1,3-benzoxazole isolated in a cryogenic argon matrix were characterized by infrared spectroscopy. The photochemistry of matrix-isolated 1,3-benzoxazole, induced by excitation with a frequency-tunable narrowband UV light, was investigated. Irradiation at 233 nm resulted in a nearly quantitative conversion of 1,3-benzoxazole into 2-isocyanophenol. The individual photochemical behavior of the in situ produced 2-isocyanophenol was studied upon excitations at 290 nm, where 1,3-benzoxazole does not react. The photochemistry of isomeric matrix-isolated 2-cyanophenol was also studied. The photoreactions of 2-substituted (cyano- or isocyano-) phenols were found to have many similarities: (i) OH bond cleavage, yielding a 2-substituted (cyano- or isocyano-) phenoxyl radical and an H-atom, (ii) recombination of the detached H-atom, resulting in an oxo tautomer, and (iii) decomposition leading to fulvenone, together with HCN and HNC. In another photoprocess, 2-cyanophenol undergoes a [1,5] H-shift from the hydroxyl group to the cyano group yielding isomeric ketenimine. The analogous [1,5] H-shift from the hydroxyl group to the isocyano group must have also occurred in 2-isocyanophenol; however, the resulting nitrile ylide isomer is kinetically unstable and collapses to benzoxazole. All photoproducts were characterized by comparing their observed infrared spectra with those computed at the B3LYP/6-311++G(d,p) level. The mechanistic analysis of the photochemistry occurring in the family of the title compounds is presented.[2]
References
[1]Lopes Jesus AJ, Nunes CM, Reva I, Pinto SMV, Fausto R. Effects of Entangled IR Radiation and Tunneling on the Conformational Interconversion of 2-Cyanophenol. J Phys Chem A. 2019 May 23;123(20):4396-4405. doi: 10.1021/acs.jpca.9b01382. Epub 2019 May 14. PMID: 30951634.
[2]Reva I, Jesus AJL, Nunes CM, Roque JPL, Fausto R. UV-Induced Photochemistry of 1,3-Benzoxazole, 2-Isocyanophenol, and 2-Cyanophenol Isolated in Low-Temperature Ar Matrixes. J Org Chem. 2021 May 7;86(9):6126-6137. doi: 10.1021/acs.joc.0c02970. Epub 2021 Apr 19. PMID: 33872502.
You may like
Lastest Price from 2-Cyanophenol manufacturers

US $50.00/kg2025-04-21
- CAS:
- 611-20-1
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000

US $0.00-0.00/KG2025-04-15
- CAS:
- 611-20-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg