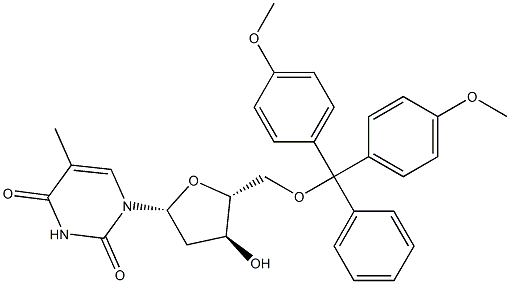
5'-O-Dimethoxytrityl-deoxythymidine: PCR Stability & Nucleic Acid Synthesis Role
5'-O-Dimethoxytrityl-deoxythymidine(DMT) is a compound found in various plants and animals that's used as a mind-altering drug. In large enough doses, DMT can give you a "high" and distort your senses so that you see or feel things that aren’t really there. Other names for DMT are Dimitri, businessman’s special, the spirit molecule, and elf spice. Various cultures have used it for hundreds of years for rituals and religious practices. 5'-O-Dimethoxytrityl-deoxythymidine is one of the active ingredients in ayahuasca, a psychedelic tea native to South America. Synthetic DMT is also made in a lab. People use the drug recreationally for the short, powerful “trip” sometimes called a “DMT breakthrough.” Some research shows it may have benefits for mental and physical health. But it can also have side effects that may outweigh any benefits. 5'-O-Dimethoxytrityl-deoxythymidine can be used in the solid-phase synthesis of polynucleotides and polythymidylic acids by the block coupling phosphotriester method. Further, it is used in the stereoselective synthesis of 3'-deoxy-3'-threo-hydroxymethyl nucleoside. In addition to this, it is used as a research tool for antiviral and anticancer studies.

5′-DMT-protected double-stranded DNA
During ON synthesis orthogonal protection systems must be used, most of which are based on acid-labile 5'-O-Dimethoxytrityl-deoxythymidine (DMT) protective group. Since its introduction as acid-labile protecting group for deoxyribonucleotide chemistry, DMT becomes a standard for the protection of the 5ʹ-OH group. Usually deblocking is realized by 2–3% trichloroacetic acid solution in toluene or dichloromethane. After last base coupling, DMT could be removed or left on the ON. Hydrophobic properties of DMT allows blocked ON to be retained on reverse-phase sorbents in the presence of trimethylamine as an ion-pairing reagent. So fast and effective procedures for ON purification based on reverse-phase solid-phase extraction cartridges were developed. During purification, 5'-O-Dimethoxytrityl-deoxythymidine is removed by washing with 2% trifluoroacetic acid. Earlier we demonstrated the ability of ZipTip microextractors to purify and dose ON pools for subsequent gene assembly. The application of DMT-ONs in standard PCR and during gene assembly was not reported earlier. Thus effectiveness of DMT-ONs for PCR amplification or for gene assembly remains unknown as well as the fate of the protective group after PCR. Further investigations of influence of bulky, lipophilic DMT-group on activity and specificity of DNA-enzymes, especially 5ʹ, 3ʹ-exonucleases, will allow outlining the prospects for practical application of 5'-O-Dimethoxytrityl-deoxythymidine dsDNA.[1]
The functionalization of 5ʹ-OH group in nucleic acids is of significant value for molecular biology. In the current work we discovered that acid-labile 5'-O-Dimethoxytrityl-deoxythymidine of oligonucleotides (ONs) is stable under PCR conditions and does not interfere with activity of DNA polymerases. So application of 5ʹ-DMT-protected ONs could allow producing both symmetric and asymmetric 5ʹ-DMT-blocked double-stranded DNA (dsDNA) fragments. We demonstrated that the presence of thiol compounds (mercaptoethanol and dithiothreitol) in PCR mixture is undesirable for the stability of DMT-group. DMT-ONs can be successfully used during polymerase chain assembly of synthetic genes. We tested 5'-O-Dimethoxytrityl-deoxythymidine dsDNA in blunt-end DNA ligation reaction by T4 DNA ligase and found that it could not be ligated with 5ʹ-phosphorylated DNA fragments, namely linearized plasmid vector pJET1.2/blunt. Possible reason for this is steric hindrance created by bulky and rigid DMT-group, that prevents entering enzyme active site. We also demonstrated that 5ʹ-DMT modification of dsDNA does not affect activity of T5 5ʹ,3ʹ-exonuclease towards both ssDNA and dsDNA. Further screening of the exonucleases, sensitive to 5'-O-Dimethoxytrityl-deoxythymidine-modification or search of ways to separate long 5ʹ-DMT-ssDNA and 5ʹ-OH-ssDNA could allow finding application of 5ʹ-DMT-modified oligo- and polynucleotides. In the current study, we confirmed that the acid-labile DMT-protecting group primarily remains on ONs in PCR conditions, pH and content of PCR buffers being essential to preserve this modification.
References
[1]Shchur VV, Burankova YP, Zhauniarovich AI, Dzichenka YV, Usanov SA, Yantsevich AV. 5'-DMT-protected double-stranded DNA: Synthesis and competence to enzymatic reactions. Anal Biochem. 2021 Mar 15;617:114115. doi: 10.1016/j.ab.2021.114115. Epub 2021 Jan 26. PMID: 33508272.
You may like
Lastest Price from 5'-O-Dimethoxytrityl-deoxythymidine manufacturers
US $0.00-0.00/G2025-06-03
- CAS:
- 40615-39-2
- Min. Order:
- 1G
- Purity:
- 98%
- Supply Ability:
- 2000
US $0.00-0.00/kg2025-04-04
- CAS:
- 40615-39-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton