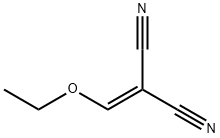
Ethoxymethylenemalononitrile: Synthesis, Pyrazole Formation & Chitosan Modification
Ethoxymethylenemalononitrile (EMMN) is an orange solid and the melting point of EMMN is 64–66 °C[1].It is an inexpensive reagent. As a functionalized malono-nitrile, it is widely used to synthesize pyrazoles, pyrimidines and a variety of fused heterocyclic systems. Triethyl orthoformate (67.3 mg, 0.454 mol) and malononitrile (20.0 mg,0.302 mol) were andded to acetic anhydride (77.2 gm, 0.75 moles) and the mixture was refluxed for 4-5 h at 110-140°C. The reaction was monitored by GC. After the reaction is complete, it is cooled to room temperature. Concentrated the mixture at 70°C at reduced pressure to yield crude solid product and the pure product (Ethoxymethylenemalononitrile) was obtained through purified either by vacuum distillation. General procedure for the synthesis of 2-(ethoxymethylene)malononitrile from triethyl orthoformate and malononitrile: Triethyl orthoformate (67.3 g, 0.454 mol) was mixed with malononitrile (20.0 g, 0.302 mol) in acetic anhydride (77.2 g, 0.75 mol), and the reaction was carried out under reflux for 4-5 hours at 110-140 °C. The reaction process was monitored by gas chromatography (GC). Upon completion of the reaction, the reaction mixture was cooled to room temperature. Subsequently, the reaction mixture was concentrated under reduced pressure at 70 °C to afford the crude product 2-(ethoxymethylene)malononitrile. The crude product was further purified by vacuum distillation to give the pure 2-(ethoxymethylene)malononitrile.

Ring formation mechanism from ethoxymethylenemalononitrile and hydrazine hydrate
Pyrazoles belong to the most important heterocycles containing nitrogen. They are classified as alkaloids, however they are rare in nature. The first natural pyrazole, 1-pyrazolyl-alanine, was isolated from the seeds of watermelons. Pyrazole derivatives have attracted considerable interest because of their long history of applications in pharmaceuticals and agrochemicals. Ethoxymethylenemalononitrile is another precursor, which gives 5-aminopyrazole-4-carbonitrile in the presence of hydrazine hydrate. The objective of this study is to provide a theoretical prediction of the kinetic and activation parameters for this reaction. It is also important to elucidate the molecular mechanism associated with this reaction in order to find out a precise idea of the reaction pathway. Experimentally, the reaction has been carried out in presence of methanol as a solvent. For better analysis of the solvent effect, our theoretical studies have been carried out in solvent and gas phases.[1]
A computational study on the kinetics and mechanism of the ethoxymethylenemalononitrile and hydrazine hydrate reaction was performed in two media. The reaction can be initiated by the nucleophilic attack of the nitrogen atom of hydrazine hydrate on the carbon atom of CC double bond of ethoxymethylenemalononitrile or on the cyanide group of hydrazine hydrate. CPCM calculations in methanol phase showed that the solvent molecules have noticeable effect on the Gibbs free energy of activation and relative Gibbs free energy of species. The nucleophilic attack of the nitrogen atom of ethoxymethylenemalononitrile to the carbon atom of the cyanide group of hydrazine hydrate is the most probable pathway in the gas and solvent phases. The calculated activation energies are too high for a process which takes place under conventional experimental conditions. It may indicate that solvent molecules or a second substrate molecule is playing an explicit role in the reaction mechanism, but this possibility is beyond the scope of this study.
Nucleophilic vinylic substitution with ethoxymethylenemalononitrile
Chitosan (Ch) is a polysaccharide obtained from chitin: a renewable, nontoxic, biocompatible biomolecule that constitutes the second most abundant natural polymer on the planet. The presence of amino groups in the chitosan polymer chains allows one to change the physicochemical properties of the natural polymer. In this work, we prepared a chitosan derivative (ChMM) with ethoxymethylenemalononitrile (EMM) through a nucleophilic vinylic substitution (SNV), changing the experimental conditions of stoichiometry, solvent (ethanol, dimethylsulfoxide (DMSO)) and reaction time (3 and 6 h). ChMM was characterized by Fourier transform infrared spectroscopy (FTIR), solid-state 13C NMR, elemental analysis (EA), thermogravimetry (TG), scanning electron microscopy (SEM) and X-ray diffraction (XRD). The FTIR and 13C NMR results confirm the success of the reaction, showing a sharp peak at 2213 cm−1 corresponding to the stretching of the C–N bond and additional signals at 52 and 166 ppm, respectively. The degree of substitution (DS) was calculated from EA and the results obtained show that sample modified with a [Ch]/[Ethoxymethylenemalononitrile] molar ratio of 1:2 over 3 h in DMSO presented the better result (DS = 0.91). ChMM did not show a considerable change in morphology (SEM) and thermal stability (TG). The insertion of methylenemalononitrile groups led to a decrease in the crystallinity index of chitosan derivatives (XRD). ChMM showed good ability to remove dyes in acidic and basic media (pH 5, 7, 9), and for crystal violet and safranin dyes the removal percentage surpassed 70%. © 2022 Society of Industrial Chemistry.[2]
References
[1] Sabet-Sarvestani, H., Eshghi, H., Bakavoli, M., Izadyar, M., & Rahimizadeh, M. (2014). Theoretical investigation of the chemoselectivity and synchronously pyrazole ring formation mechanism from ethoxymethylenemalononitrile and hydrazine hydrate in the gas and solvent phases: DFT, meta-GGA studies and NBO analysis†. RSC Advances, 82, 43485–43495.
[2] Robson V Pereira. (2022). A new route to produce chitosan derivatives: nucleophilic vinylic substitution with ethoxymethylenemalononitrile. Polymer International, 72 3, 376–382.
You may like
Lastest Price from Ethoxymethylenemalononitrile manufacturers

US $0.00/Kg2023-04-26
- CAS:
- 123-06-8
- Min. Order:
- 1Kg
- Purity:
- 99.0% up
- Supply Ability:
- 50 tons per month

US $0.00/KG2022-02-22
- CAS:
- 123-06-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 100 tons