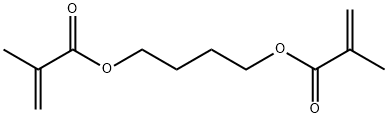
1,4-Butanediol Dimethacrylate: Cell Effects & Glove Permeability
1,4-Butanediol Dimethacrylate, also known as Tetramethylene dimethacrylate, is a versatile ingredient commonly used in the cosmetic industry. Chemically, it is a diester of methacrylic acid and 1,4-butanediol. This compound is known for its ability to form films, making it a valuable component in various cosmetic formulations. The history of 1,4-Butanediol Dimethacrylate in cosmetics dates back to the mid-20th century when chemists began exploring synthetic polymers for their unique properties. Initially used in industrial applications, its potential in cosmetics was soon realized due to its film-forming capabilities. Over the years, it has become a staple in products that require a durable, flexible film on the skin or hair.

Effects of 1,4-butanediol dimethacrylate on HL-60 cell metabolism
The polymerization of methacrylates present in dental composite resins is never complete, and when monomers such as triethyleneglycol dimethacrylate (TEGDMA), 2-hydroxyethylmethacrylate (HEMA), bisphenol A glycerolate (1 glycerol/phenol) dimethacrylate (Bis-GMA), 1,4-butanediol dimethacrylate (BDDMA), urethane dimethacrylate (UDMA), or others are converted to the high-molecular-weight networked solid, residual unreacted monomers remain trapped in the structure. The incomplete conversion causes, along with a reduction in the mechanical strength, the release of monomers, the presence of which may result in adverse effects in the organism. As the intracellular mechanisms of the aforesaid effects are still not completely clear, we decided to investigate the biochemical interactions between methacrylates and human cells. In particular, we studied the behaviour of 1,4-Butanediol dimethacrylate and UDMA because their cytotoxicity and the mechanisms underlying it are poorly known. To avoid interference owing to synergistic and/or antagonistic effects often observed in methacrylate mixtures, we chose to employ pure monomer compounds. We therefore decided to investigate the cell-differentiating effects of UDMA and 1,4-Butanediol dimethacrylate monomers and their biochemical effects. In fact, if, on the one hand, it could be expected that the presence of these compounds resulted in effects quite similar to the other methacrylates already studied, on the other hand non-overlapping behaviours could also derive from their structural diversity. [1]
Dental composite resins have been employed worldwide since the mid-1950s for adult and young patients: a careful evaluation of the interactions between the components of these materials and the host is therefore mandatory. In this study, experiments on proliferation and differentiation have been performed to select monomer concentrations (0.4 mmol l−1 of 1,4-Butanediol dimethacrylate and 55 mmol l−1 of UDMA) capable of inducing cell differentiation, albeit concurrently with an increased cell mortality, probably through a non-specific receptor-less way. Both 1,4-Butanediol dimethacrylate and UDMA induce an increase of cellular lactate without altering G6PDH activity, implying that glucose is disposed of mainly through anaerobic glycolysis to produce energy, whereas G6PDH and glutathione reductase activities are unaffected, thus indicating that no increase of NADPH production is required. Enhanced ROS production has been found in other tissues undergoing HEMA treatment (i.e. human gingival fibroblasts), although the source or cause of ROS could not be identified. By contrast, 1,4-Butanediol dimethacrylate and UDMA monomers have not been found to provoke an increase of ROS production in HL-60 cells. We cannot conclude whether the lack of ROS formation can be ascribed to the small number of mitochondria possessed by this cell line compared with gingival fibroblasts or to the structural differences among methacrylic monomers.
Permeability of different types of medical protective gloves to acrylic monomers
Dental restorative materials such as dentures and temporary restorations, as well as orthopedic bone cement, are composed of poly (methyl methacrylate) (PMMA) and a monomer liquid. The main constituent of the monomer liquid is methyl methacrylate (MMA). Dental materials may also contain cross-linking agents such as ethylene glycol dimethacrylate (EGDMA) or 1,4-butanediol dimethacrylate (1,4-BDMA), in order to improve mechanical properties and resistance crazing. Handling of acrylic monomers constitutes an important health hazard for dental personnel. Patch tests of dental personnel with allergies have shown a positive response to MMA , EGDMA and 1,4-Butanediol dimethacrylate. The aim of this study was to determine breakthrough time (BTT) and use this as a measure of the protection (according to the European Standard EN 274-3:1994) afforded by different types of medical protective gloves against permeation by MMA, EGDMA and 1,4-BDMA in a mixture.[2]
Size and structure of the monomer, are important with regard to permeation of monomers. As expected, the smallest monomer, MMA, permeated earliest followed by EGDMA and 1,4-Butanediol dimethacrylate. Dental materials never consist of a single monomer, which makes it necessary to test a mixture of monomers representing commonly used dental prosthetic materials. The present investigation showed that in the presence of MMA, the permeation of both EGDMA and 1,4-BDMA was enhanced compared with testing only EGDMA and 1,4-Butanediol dimethacrylate. The enhanced permeation rate may be due to MMA acting as a vehicle for the larger monomers. When testing the Metin glove using a mixture without MMA, the glove lasted the whole test period and provided better protection to EGDMA and 1,4-BDMA than the natural rubber latex Sempermed. Clinically, destruction of glove material by monomer contact is of interest because destruction enhances permeation of monomers and microorganisms through the gloves.
References
[1]Nocca, Giuseppina et al. “Effects of 1,4-butanediol dimethacrylate and urethane dimethacrylate on HL-60 cell metabolism.” European journal of oral sciences vol. 117,2 (2009): 175-81. doi:10.1111/j.1600-0722.2008.00606.x
[2]Lönnroth, Emma-Christin et al. “Permeability of different types of medical protective gloves to acrylic monomers.” European journal of oral sciences vol. 111,5 (2003): 440-6. doi:10.1034/j.1600-0722.2003.00064.x
You may like
Lastest Price from 1,4-Butanediol dimethacrylate manufacturers

US $50.00-10.00/kg2025-11-03
- CAS:
- 2082-81-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 600tons

US $0.00/Kg/Drum2025-04-21
- CAS:
- 2082-81-7
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 10 tons