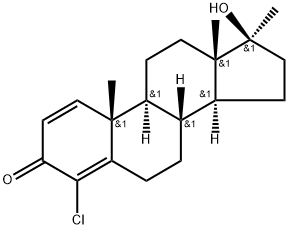
4-Chlorodehydromethyltestosterone: From Development and Abuse to Advanced Detection Strategies
4-Chlorodehydromethyltestosterone is an anabolic-androgenic steroid that was developed by Jenapharm in the 1960s and was marketed as Oral Turinabol®. It is prohibited in sports at all times. Even if discontinued as pharmaceutical in 1994, there are several adverse analytical findings by anti-doping laboratories every year.

Controlled administration of 4-Chlorodehydromethyltestosterone in humans
An annual list of prohibited substances and methods is published by the World Anti-Doping Agency (WADA). In addition to growth factors, hormones, and masking substances such as diuretics, which are prohibited at all times, the list also includes cannabinoids and narcotics, for example, which are only banned during competition. Anabolic substances belong to the first group and are therefore prohibited both in and out of competition. Most of them are derivatives of the steroid testosterone and mediate their effect via agonism at the androgen receptor. 4-Chlorodehydromethyltestosterone (4-chloro-17β-hydroxy-17α-methyl-androsta-1,4-dien-3-one, DHCMT) is an anabolic-androgenic steroid which was introduced into the market as “Oral-Turinabol” by East German pharmaceutical company Jenapharm in the 1960s. It is a derivative of testosterone with enhanced anabolic properties which is orally bioavailable due to its alkylation at C-17. According to Zafferoni et al. the chlorination at C-4 does not affect the anabolic or androgenic activity of 4-Chlorodehydromethyltestosterone, which is contrary to the activity of progestogens or corticoids after substitution in this position. 4-Chlorodehydromethyltestosterone was primarily developed to support recovery after major surgeries. However, shortly after its introduction on the market, it was misused in sports as performance-enhancing drug in one of the biggest systematic doping programs, until the German Democratic Republic’s collapse.[1]
4-Chlorodehydromethyltestosterone was sold in two dosages, 1 and 5 mg, with recommendations for daily intake up to 50 mg. It is explicitly mentioned as anabolic androgenic steroid (AAS) in class S1 in the list of prohibited substances published by the World Anti-Doping Agency (WADA) every year. Even if discontinued as approved drug, the substance is still misused in sports until today. Due to interindividual differences there were several varieties in the excretion profiles among the volunteers. The metabolite M3, which has a fully reduced A-ring and modified D-ring structure, was identified by comparison with reference material as 4α-chloro-17β-hydroxymethyl-17α-methyl-18-nor-5α-androstan-13-en-3α-ol. It was found to be suitable as long-term marker for the intake of 4-Chlorodehydromethyltestosterone in four of the volunteers. In one of the volunteers, it was detectable for 45 days after single oral dose administration. However, in two of the volunteers M5 (already published as long-term metabolite in the 1990s) showed longer detection windows. In one volunteer M3 was undetectable but another metabolite, M2, was found as the longest detectable metabolite. It is highly recommended to screen for all known metabolites in both fractions, glucuronidated and unconjugated, to improve identification of cheating athletes. This study also offers some deeper insights into the metabolism of 4-Chlorodehydromethyltestosterone and of 17α-methyl steroids in general.
Detection and characterization of 4-Chlorodehydromethyltestosterone
The anabolic androgenic steroids (AAS) are the class of performance enhancing substances prohibited in sports by the World Anti-Doping Agency (WADA) at all times. Due to their availability on the black market, some AAS are more likely to be abused than the others. One of such substances is 4-Chlorodehydromethyltestosterone (DHCMT), also known as 4-chloromethandienone or oral-turinabol, a steroid which is remarkable, inter alia, for its relatively fast elimination rate from the body. The data on the metabolism of DHCMT are limited. In 1970 Schubert et al. reported the detection of parent steroid, as well as 6β-hydroxy-, 16β-hydroxy- and 6β,16-dihydroxy-DHCMT in post administration urine. Afterward, Dürbeck et al. identified 6β-hydroxy-, 6β,12-dihydroxy-, 6β,16-dihydroxy-DHCMT and minor amounts of epi-DHCMT, but did not detect parent drug nor 16β-hydroxy-DHCMT. The presence of 6β-hydroxy-DHCMT in human urine was also confirmed. However, the detection window was quite small (5 days or less). Later, an in-depth study was performed that revealed a high complexity of the 4-Chlorodehydromethyltestosterone metabolism and resulted in the identification and characterization of the other important metabolites.[2]
The biotransformation of 4-Chlorodehydromethyltestosterone (DHCMT, 4-chloro-17β-hydroxy,17α-methylandrosta-1,4-dien-3-one) in man was studied with the aim to discover long-term metabolites valuable for the antidoping analysis. Having applied a high performance liquid chromatography for the fractionation of urinary extract obtained from the pool of several DHCMT positive urines, about 50 metabolites were found. Most of these metabolites were included in the GC–MS/MS screening method, which was subsequently applied to analyze the post-administration and routine doping control samples. Using GC–MS and GC–MS/MS the most important metabolite for the antidoping analysis was tentatively characterized as 4-chloro-18-nor-17β-hydroxymethyl,17α-methyl-5β-androst-13-en-3α-ol. This metabolite together with its 17α-epimer was shown to provide much better detectability of 4-Chlorodehydromethyltestosterone abuse for extended period of time compared to the other known metabolites. The synthesis of reference steroids is needed to confirm the proposed structure of the identified metabolites. The most long-term metabolite was shown to be superior in the majority of cases to the other known 4-Chlorodehydromethyltestosterone metabolites, such as 4-chloro-18-nor-17β-hydroxymethyl,17α-methylandrosta-1,4,13-trien-3-one and 4-chloro-3α,6β,17β-trihydroxy-17α-methyl-5β-androst-1-en-16-one.
References
[1]Loke, Steffen et al. “Controlled administration of dehydrochloromethyltestosterone in humans: Urinary excretion and long-term detection of metabolites for anti-doping purpose.” The Journal of steroid biochemistry and molecular biology vol. 214 (2021): 105978. doi:10.1016/j.jsbmb.2021.105978
[2]Sobolevsky, Tim, and Grigory Rodchenkov. “Detection and mass spectrometric characterization of novel long-term dehydrochloromethyltestosterone metabolites in human urine.” The Journal of steroid biochemistry and molecular biology vol. 128,3-5 (2012): 121-7. doi:10.1016/j.jsbmb.2011.11.004
Lastest Price from 4-Chlorodehydromethyltestosterone manufacturers

US $400.00/g2025-11-24
- CAS:
- 2446-23-3
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 999

US $10.00/box2025-10-13
- CAS:
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 9999