The influence of N-isopropyl-N-phenyl-1,4-phenylenediamine on environment
Introduction
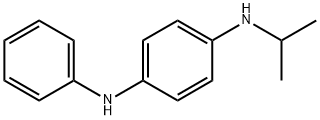
N-Isopropyl-N'-phenyl-1,4-phenylenediamine (IPPD;Figure 1) is used as an antioxidant in rubber, mainly for car tire, and the annual production of IPPD in the world is 10,000–15,000 tonnes. The log octanol-water partition coefficient (log Kow) value of IPPD is 3.88, and the half-life values of N-isopropyl-N'-phenyl-1,4-phenylenediamine had been measured in waters of various degrees of purity from 2 to 16 h. Rare study has paid attention to the toxicity of N-isopropyl-N'-phenyl-1,4-phenylenediamine, and the level of it in environment is unclear. A recent study showed that the level of IPPD in runoff water of Hong Kong varied in the range of not detected -2.43μg/L.

To date, the information about the toxicity of N-isopropyl-N'-phenyl-1,4-phenylenediamine is rare. There is no data on the effects of IPPD following acute inhalation or oral exposure in humans. In two briefly reported studies showed that N-isopropyl-N'-phenyl-1,4-phenylenediamine is of moderate toxicity by the oral route in rats, and of very low toxicity by the dermal route in rabbits.That IPPD is of moderate toxicity by the oral route in rats, and of very low toxicity by the dermal route in rabbits. IPPD is a skin sensitizer to animal and human. In vitro mammalian cell mutagenicity assays showed that N-isopropyl-N'-phenyl-1,4-phenylenediamine had a potential to induce chromosome aberrations. According to the acute toxicity results, the 96 h lethal concentration 50 (LC50) for bluegill sunfish, rainbow trout and fathead minnow ranged from 0.34 to 0.43 mg/L. And no information is available on the sub-acute effects of IPPD on fish. Whether it has obvious developmental toxicity to aquatic organisms is unclear.[1]
Effect on zebrafish of N-isopropyl-N-phenyl-1,4-phenylenediamine
N-isopropyl-N-phenyl-1,4-phenylenediamine (IPPD) is used as a ubiquitous antioxidant worldwide, it is an additive in tire rubber easily discharged into the surrounding environment. At present, there is no study concerning the subacute toxicity of N-isopropyl-N-phenyl-1,4-phenylenediamine on fish. We used zebrafish embryos (2h post-fertilization) exposed to IPPD for 5days at concentrations of 0, 0.0012, 0.0120 and 0.1200 mg/L to investigate its toxic effects of embryonic development, disruption of growth hormone/insulin-like growth factor (GH/IGF) and hypothalamic-pituitarythyroid (HPT) axis. The results showed that IPPD exposure decreased hatchability, weakened movement ability, reduced body length, and caused multiple types of deformities in zebrafish embryos. The expression of genes involved to GH/IGF and HPT axis were altered after exposure to N-isopropyl-N-phenyl-1,4-phenylenediamine in zebrafish larvae. Meanwhile, exposure to N-isopropyl-N-phenyl-1,4-phenylenediamine significantly decreased thyroxine (T4) and 3,5,3′-triiodothyronine (T3) contents in larvae, which indicated that HPT axis was in a disturbed state. Moreover, treatment of N-isopropyl-N-phenyl-1,4-phenylenediamine decreased the enzymatic activities of superoxide dismutase (SOD) and catalase (CAT) as well as levels of glutathione (GSH). While the contents of malondialdehyde (MDA) were elevated after exposure to IPPD. The present study thus demonstrated that IPPD induced oxidative stress, caused developmental toxicity and disrupted the GH/IGF and HPT axis of zebrafish,which could be responsible for developmental impairment and growth inhibition.[1]
Aquatic Thermal and Photochemical Reactivity of N-isopropyl-N'-phenyl-1,4-phenylenediamine
A ubiquitously used tire rubber antidegradant, 6PPD (N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylenediamine), and its toxic ozonation product, 6PPD-quinone (N-(1,3-dimethylbutyl)-N'-phenyl-p-phenylenediamine quinone), have become recognized as important environmental pollutants since 6PPD-quinone (6PPD-Q) was identified as the likely cause of decades of mass Coho salmon kills. The reactivity of 6PPD, 6PPD-Q, and similar phenylenediamines requires study to better understand their environmental fate. This study explores the aquatic reactivity of 6PPD, N-isopropyl-N'-phenyl-1,4-phenylenediamine (IPPD), and 6PPD-Q through thermal and photochemical pathways using both steady-state photochemistry and time-resolved laser spectroscopy techniques. 6PPD was found to rapidly degrade in the dark, with its degradation rate being highly dependent on the pH, temperature, and oxygen concentrations. IPPD behaves similarly to 6PPD. In contrast, 6PPD-Q is much more stable in the dark. All three chemicals are degraded via direct photochemistry. Regarding indirect photochemistry, 3CDOM plays a role in the degradation of 6PPD and N-isopropyl-N'-phenyl-1,4-phenylenediamine but not 6PPD-Q, while 1O2 does not play a significant role for any of the compounds. Reaction rate constants are reported as well as 6PPD-Q molar yields from 6PPD, which were minimal for all aqueous pathways examined. 6PPD-Q may have a longer environmental lifetime as there are fewer degradation pathways. This research will help us to better understand and control these chemicals in the environment.[2]
References
1.Zhong L, Peng W, Liu C, Gao L, Chen D, Duan X. IPPD-induced growth inhibition and its mechanism in zebrafish. Ecotoxicol Environ Saf. 2022;239:113614. doi:10.1016/j.ecoenv.2022.113614
2.Platt KL, Yushchenko O, Laszakovits JR, Zhang Y, Pflug NC, McNeill K. Aquatic Thermal and Photochemical Reactivity of N-(1,3-Dimethylbutyl)-N'-phenyl-p-phenylenediamine (6PPD), N-Isopropyl-N'-phenyl-p-phenylenediamine (IPPD), and 6PPD-quinone. Environ Sci Technol. 2025;59(25):12900-12909. doi:10.1021/acs.est.4c12896
You may like
Lastest Price from N-Isopropyl-N'-phenyl-1,4-phenylenediamine manufacturers

US $6.00/kg2025-04-21
- CAS:
- 101-72-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/KG2025-04-15
- CAS:
- 101-72-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg