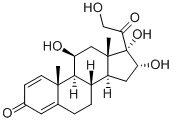
Exploring 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione: Advances in Synthesis, Biotechnological Conversion, and Drug Metabolism
Introduction
16α-Hydroxyprednisolone (molecular formula C₂₁H₂₈O₆), chemically named 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione, is a non-halogenated steroidal glucocorticoid and an important pharmaceutical intermediate. It serves as a key raw material for the synthesis of glucocorticoid drugs such as budesonide, ciclesonide, and desonide. These non-halogenated glucocorticoids are widely used in the treatment of refractory asthma and inflammatory diseases. In particular, budesonide and ciclesonide offer distinct advantages, including low dosage requirements, strong local anti-inflammatory effects, and minimal systemic side effects, making them especially suitable for pediatric use. Consequently, they are considered first-line therapies for severe asthma and allergic rhinitis. As the essential precursor for the production of these glucocorticoids, 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione holds significant market potential.
Biotransformationof 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione, an anti-inflammatory drug, could be potentially obtained from hydrocortisone bioconversion by combining a 1,2-dehydrogenation reaction performed by Arthrobacter simplex ATCC31652 with a 16α-hydroxylation reaction by Streptomyces roseochromogenes ATCC13400. The A. simplex capability to 1,2-dehydrogenate the 16α-hydroxyhydrocortisone, the product of S. roseochromogenes transformation of hydrocortisone, and vice versa the capability of S. roseochromogenes to 16α-hydroxylate the prednisolone were assessed. Bioconversions were studied in shake flasks and strain morphology changes were observed by SEM. Whole cell experiments were set up to perform the two reactions in a sequential mode in alternate order or contemporarily at diverse temperature conditions. A. simplex catalyzed either the dehydrogenation of hydrocortisone into prednisolone efficiently or of 16α-hydroxyhydrocortisone into 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione in 24 h (up to 93.9%). Surprisingly S. roseochromogenes partially converted prednisolone back to hydrocortisone. A 68.8% maximum of 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione was obtained in 120-h bioconversion by coupling whole cells of the two strains at pH 6.0 and 26 ◦C. High bioconversion of hydrocortisone into 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione was obtained for the first time by coupling A. simplex and S. roseochromogenes. [1]
Ionic Synthesis of 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione
This invention presents an efficient and environmentally friendly method for the synthesis of 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione, in which an acidic ionic liquid is employed simultaneously as the reaction medium and catalyst. Compared with conventional synthetic procedures, the method offers several advantages, including simple operation, high productivity, improved efficiency, reduced formation of the “three wastes” (wastewater, waste gas, and solid residues), and convenient product isolation. Moreover, the ionic liquid can be recycled and reused multiple times without significant loss of activity, thereby enhancing the economic feasibility and sustainability of the process. In thprednisolone (formula I) is used as the starting substrate. First, it undergoes a ↳dehydration reaction in the acidic ionic liquid described by formula II, which generates a double bond. Subsequently, an aqueous solution of hydrogen peroxide is added as an oxidant to promote the oxidation reaction. After completion, water is introduced to hydrolyze the intermediate products, ultimately yielding the desired compound, 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione. In formula (II), R represents a C1–C10 substituted alkyl group bearing sulfonic or carboxyl substituents, and L can be selected from HSO₄, COO, CH₃COO, BF₄, PF₆, or CF₃SO₃; alternatively, R may be an unsubstituted C1–C10 alkyl group with L restricted to HSO₄, COO, or CF₃SO₃. This process thus establishes a green, economical, and scalable route for producing 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione, a key intermediate in non-halogenated glucocorticoid synthesis. [2]

11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione as a Metabolite
Budesonide is a synthetic glucocorticosteroid that is commonly used in topical treatment of asthma and rhinitis. The main metabolites formed from budesonide in human liver microsomes have been identified as 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione and 6 beta-hydroxy-budesonide. The study found a strong correlation between formation of the two main metabolites and testosterone 6 beta-hydroxylation (correlation 0.98 and 0.95), a marker for CYP3A. When budesonide (10 microM) was incubated with human liver microsomes in the presence of compounds known to interact with different isoforms or subfamilies of CYP, ketoconazole was found to be the strongest inhibitor of budesonide metabolism (IC50: approximately 0.1 microM) followed by troleandomycin (IC50: approximately 1 microM), erythromycin, and cyclosporin, all substances known to interact with CYP3A isoenzymes. Substances known to interact with CYP2C (sulfaphenazole, mephenytoin, and tolbutamide) and with CYP2D6 (bufuralol and quinidine) did not specifically inhibit the metabolism of budesonide. In addition, formation of the budesonide metabolites (11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione and 16 beta-hydroxybudesonide) was inhibited by antibodies against the CYP3A subfamily, but not by antibodies against the CYP1A subfamily or control immunoglobulin G. The study conclude that the formation of 11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione and 6 beta-hydroxybudesonide from budesonide is catalyzed by isoenzymes within the CYP3A subfamily. [3]
References:
[1] Restaino, O. F., Barbuto Ferraiuolo, S., Perna, A., Cammarota, M., Borzacchiello, M. G., Fiorentino, A., & Schiraldi, C. (2020). Biotechnological transformation of hydrocortisone into 16α-hydroxyprednisolone by coupling Arthrobacter simplex and Streptomyces roseochromogenes. Molecules, 25(21), 4912.
[2] Zhejiang University of Technology. (2012, August 8). A method for synthesizing 16α-hydroxyprednisolone (CN201010205878.0) [Patent].
[3] J?nsson, G., Astr?m, A., & Andersson, P. (1995). Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug metabolism and disposition, 23(1), 137-142.
Lastest Price from 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 13951-70-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton

US $0.00-0.00/Box2025-04-12
- CAS:
- 13951-70-7
- Min. Order:
- 1Box
- Purity:
- 0.99
- Supply Ability:
- 3000box