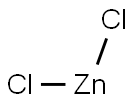Learn More About Zinc Chloride
Zinc Chloride is an ionic salt essential for the synthesis of cholesterol, protein, and fats.

Physical Properties
Zinc chloride is solid at room temperature and has a white crystalline appearance. It is odourless.
The solubility of this compound in water corresponds to 432g/100g. It is also soluble in acetone, ethanol, and glycerol.
The four polymorphs of ZnCl2 feature a tetrahedral coordinate geometry between the Zn2+ ions and the Cl–
Molten zinc chloride is deeply viscous and has a relatively low electrical conductivity value.
Chemical Properties
When ZnCl2 is dissolved in water, the resulting solution is acidic in nature. The pH of an aqueous solution of zinc chloride with a concentration of 6M is 1.
This compound reacts with ammonia to form complexes. Examples include Zn(NH3)4Cl2 and ZnCl2(NH3)2.
When heated, the hydrated form of zinc chloride loses water and minor quantities of ZnCl(OH) are obtained.
The chemical equation for this reaction is given by: ZnCl2.2H2O → ZnCl(OH) + H2O + HCl
Toxicity
Toxicity Zinc chloride is corrosive by ingestion and highly irritant by inhalation. Features Topical - Topical zinc chloride causes ulceration and burns and chronic exposure has been associated with anorexia, fatigue and weight loss.
You may like
Related articles And Qustion
See also
Lastest Price from Zinc chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7646-85-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $0.00/Kg/Bag2025-04-15
- CAS:
- 7646-85-7
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 10 tons