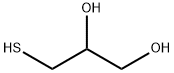
Application of 3-Mercapto-1,2-propanediol
General description
3-Mercapto-1,2-propanediol is a fine chemical. 1-thioglycerol can be used to prepare a kind of inorganic porous nanomaterial and a kind of nano-sulfide near red foreign afterglow material.
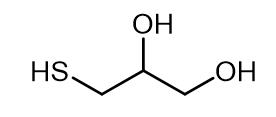
Fig. 1 The structure of 3-Mercapto-1,2-propanediol.
Application
Protected gold nanoparticles
Monolayer protected gold nanoparticles (MPCs) are the focus of recent research for their stability and are deemed as the building blocks of bottom-up strategies. In this Letter, 3-mercapto-1,2-propanediol monolayer protected gold nanoparticles (MPD-MPCs) were synthesized and characterized by transmission electron microscopy, UV/Vis spectroscopy, X-ray photoelectron spectroscopy and Fourier transform infrared spectroscopy. The value of quantized double-layer capacitance (1.13 aF) of MPD-MPCs in aqueous media was obtained by differential pulse voltammograms [1].
As replacing agent
A simple, rapid and selective complexometric method is proposed for the determination of mercury(ll). Mercury(II) along with other lolls is first complexed with excess of EDTA and the surplus EDTA is back titrated (pH 5-6, hexamine buffer) with standard zinc sulphate solution (xylenol orange as indicator). 3-Mercapto-1,2-propanediol is then added to displace EDTA selectively from the Hg-EDTA complex and the released EDTA is titrated with standard zinc sulphate solution. Reproducible and accurate results are obtained in the range 3-75 mg of mercury with a relative error +/- 0.41% and coefficient of variation (n = 6) not exceeding 0.43%. The method is used for the estimation of mercury in complexes and in synthetic mixtures [2].
Ketalizations of aryl omega-(2-imidazolyl)alkyl ketones
Aryl 2-[(2-imidazolyl)ethyl or 3-(2-imidazolyl)propyl]ketones were ketalized by glycerol or 3-mercapto-1,2-propanediol in boiling benzene in the presence of 4-toluenesulfonic acid to provide the title compounds. The aryl substituents are 4-chloro-, 4-bromo-, 4-fluoro-, or 2,4-dichlorophenyl. While aryl (2-imidazolyl)methyl ketones condensed with glycerol to form cis- and trans-{2-aryl-2-[(2-imidazolyl)methyl]-4-(hydroxymethyl)}-1,3-dioxolanes, related condensations with 3-mercapto-1,2-propanediol, under similar, or even more stringent reaction conditions, produced no 1,3-oxathiolane analogs, with the starting ketones being recovered. Separation and structure determination of these racemic cis and trans isomeric products are described. The structure of these stereoisomers was established by means of H-1 and C-13 nmr correlation and nOe experiments. Selective methylation of the N-unsubstituted 2-imidazolyl alcohols with one equivalent sodium hydride and methyl iodide provided the corresponding N-methyl alcohols in excellent yields. With excess benzoyl chloride, N-unsubstituted 2-imidazolyl alcohols were initially converted to O,N-dibenzoates from which the N-benzoyl group was easily cleaved by ammonium hydroxide in ethanol to provide benzoate esters [3].
Preparation of polyaniline/Ag nanocomposites
PANI/Ag composite particles were prepared through two-step reaction process comprising the reduction of the PANI backbone by the substitution of 3-mercapto-1,2-propanediol (MPD) and the simultaneous redox reaction between AgNO3 and MPD-substituted PANI. In order to investigate the effects of MPD and AgNO3 on the oxidation state of PANI, MPD and AgNO3 were sequentially added into PANI/NMP solution, and the oxidation state of PANI was investigated by time-dependent UV-vis spectra. On the basis of UV-vis spectroscopic study, MPD-substituted PANI/Ag composite was prepared through following two steps: (1) treating PANI particles with MPD (reduction of PANI backbone); (2) adding AgNO3 solution to MPD-substituted PANI (re-oxidation of PANI backbone). The molar ratio of AgNO3 to aniline unit in the PANI chains was varied from 1: 16 to 1:1. The substitution of PANI with MPD and the formation of PANI/Ag composite were characterized by FT-IR, XRD and EDX The morphologies of PANI/Ag composites were investigated by TEM [4].
Preparation of achiral Janus nanoparticles
Chiral nanostructures have been attracting extensive interest in recent years primarily because of the unique materials properties that can be exploited for diverse applications. In this study, gold Janus nanoparticles, with hexanethiolates and 3-mercapto-1,2-propanediol segregated on the two hemispheres of the metal cores (dia. 2.7 +/- 0.4 nm), self-assembled into vesicle-like, hollow nanostructures in both water and organic media, and exhibited apparent plasmonic circular dichroism (PCD) absorption in the visible range. This was in contrast to individual Janus nanoparticles, bulk-exchange nanoparticles where the two ligands were homogeneously mixed on the nanoparticle surface, or nanoparticles capped with only one kind of ligand. The PCD signals were found to become intensified with increasing coverage of the 3-mercapto-1,2-propanediol ligands on the nanoparticle surface. This was accounted for by the dipolar property of the structurally asymmetrical Janus nanoparticles, and theoretical simulations based on first principles calculations showed that when the nanoparticle dipoles self-assembled onto the surface of a hollow sphere, a vertex was formed which gave rise to the unique chiral characteristics. The resulting chiral nanoparticle vesicles could be exploited for the separation of optical enantiomers, as manifested in the selective identification and separation of d-alanine from the l-isomer [5].
References
[1] Li D, Li J. Preparation, characterization and quantized capacitance of 3-mercapto-1, 2-propanediol monolayer protected gold nanoparticles[J]. Chemical physics letters, 2003, 372(5-6): 668-673.
[2] Shetty P, Khader A M A, Shetty A N, et al. Complexometric determination of mercury (II) with 3-mercapto-1, 2-propanediol as replacing agent[J]. REVUE ROUMAINE DE CHIMIE, 1995, 40(4): 351-355.
[3] Kim J W, Davis F S, Huang L F, et al. Ketalizations of aryl ω‐(2‐imidazolyl) alkyl ketones by glycerol and 3‐mercapto‐1, 2‐propanediol. synthesis and characterization of cis‐and trans‐{2‐aryl‐2‐[ω‐(2‐imidazolyl) alkyl](1, 3‐dioxolan‐4‐yl and 1, 3‐oxathiolan‐5‐yl)} methanols[J]. Journal of heterocyclic chemistry, 1996, 33(1): 65-74.
[4] Lee Y, Park J, Jun Y, et al. 3-Mercapto-1, 2-propanediol-substituted polyaniline/Ag nanocomposites prepared by concurrent reduction and substitution chemistry[J]. Synthetic metals, 2008, 158(3-4): 143-149.
[5] Lu J E, Yang C H, Wang H, et al. Plasmonic circular dichroism of vesicle-like nanostructures by the template-less self-assembly of achiral Janus nanoparticles[J]. Nanoscale, 2018, 10(30): 14586-14593.
You may like
Related articles And Qustion
See also
Lastest Price from 3-Mercapto-1,2-propanediol manufacturers

US $10.00/KG2025-04-21
- CAS:
- 96-27-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $1.10/g2025-04-17
- CAS:
- 96-27-5
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min