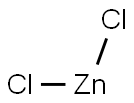Is zinc chloride safe for humans?
Description
Zinc chloride has the empirical formula Cl2Zn and a molecular weight of 136.29. It forms white, odorless, deliquescent granules with a density of 2.907. It has a melting point of approximately 290°C and a boiling point of 732°C. One gram of zinc chloride dissolves in 0.5 ml water, 1.3 ml ethanol, or 2 ml glycerol. The compound is freely soluble in acetone. Zinc oxychloride is formed in the presence of water.

Health hazard research
Zinc chloride is generally caustic, and inhalation of zinc chloride fume occurs mainly via exposure to military smoke bombs. A review of 30 case reports testifies to the severity of illness. For example, two soldiers exposed briefly had minor symptoms for 10 days and then rapidly deteriorated with severe respiratory distress, pulmonary hypertension, and death on the 25th and 32nd days. Pathology in their lungs included interstitial and intraalveolar fibrosis, endothelial proliferation, and vascular occlusions. Additionally, 13 soldiers were exposed for no longer than 5–10 min to smoke from smoke bombs in the open air or to smoke that entered a house. At first, there were few complaints, and all were treated intravenously with hydrocortisone. The four believed to have been exposed for more than 1 minute were treated systemically with hydrocortisone for 1 month: during the first 4 weeks, their lung carbon monoxide diffusion capacity decreased substantially. Eight or more weeks later, they continued to complain of respiratory symptoms.
To touch
Harmful if swallowed
Causes severe skin burns and eye damage
Causes serious eye damage
It may cause respiratory irritation
Very toxic to aquatic life with long-lasting effects. Zinc Chloride is a severe marine pollutant that may cause long-term adverse effects on the aquatic environment.
Zinc chloride is corrosive by ingestion and highly irritant by inhalation. Topical zinc chloride causes ulceration and burns, and chronic exposure has been associated with anorexia, fatigue, and weight loss.
In mouthwash
Water-soluble Zinc salts, including other Zinc Acetate and Zinc Chloride with the exception of Zinc 4-hydroxy benzene sulphonate and Zinc Pyrithione, are regulated in entry 24 of Annex III of Regulation (EC) 1223/2009. They are currently allowed for use in cosmetic products in concentrations of up to 1% as Zinc with a wide range of functions ranging from antioxidant to antimicrobial and skin protecting.
The SCCS has estimated that exposure to water-soluble zinc salts via toothpaste and mouthwash at 1 and 0.1%, respectively, may lead to a daily intake level of 3.54 mg for adults and children aged 6-17 years. This exposure constitutes between 14 and 35% of the Upper Limit (UL) for these age groups. Therefore, the SCCS considers that using Zinc in toothpaste and mouthwash per se is safe for adults and children aged 6-17 years. The SCCS has estimated that exposure to water-soluble zinc salts via toothpaste at concentrations of 1% may lead to a daily intake level of 1.0-2.00 mg for children aged 0.5-5 years. This exposure constitutes between 10 and 29% of the UL for this age group. Therefore, the SCCS considers that using Zinc in toothpaste per se is safe for children aged 0.5-5 years.
You may like
Related articles And Qustion
See also
Lastest Price from Zinc chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7646-85-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $0.00/Kg/Bag2025-04-15
- CAS:
- 7646-85-7
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 10 tons