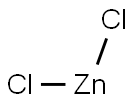Uses and Side effects of Zinc chloride
Zinc chloride is the name of chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water.ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from sources of moisture, including the water vapor present in ambient air.

Uses
Used as dehydrating and condensing agent in organic synthesis industry and catalyst for production of vanillin, Cyclamen aldehyde, anti-inflammatory painkillers and cation exchange resin;
Used as the raw material for the production of fiber can and shuttle(cosolvent for cotton fibre) to improve the fiber adhesion force;
Used as stabilizers for ice dye chromogenic salt in Dye industry in the production of reactive dye and cationic dye;
Used for impregnating wood to provide corrosion resistance and flame retardancy; Used as flame retardant for cardboard and cloth products;
Used as welding flux for welding electrode;
Used for the production of aluminum alloy, light metal deacidification and the processing of metal surface oxide layer in Metallurgical industry;
Used as the raw material for the production of Alcohol resistant foam extinguishing agent and zinc cyanide.
Also used in medicine and medicine production.
Safety
Zinc chloride is a skin irritant. After contact of the skin, immediate removal is necessary using soap and plenty of water. After contact of the eyes, adequate measures are rinsing with plenty of water or other eye rinse and contacting an ophthalmologist as soon as possible.
Personal protection: particulate filter respirator adapted to the airborne concentration of the substance. Do NOT let this chemical enter the environment. Sweep spilled substance into covered containers. Carefully collect remainder. Then store and dispose of according to local regulations.
Side effects
Zinc chloride is caustic to the gastrointestinal tract, occasionally leading to hematemesis. Symptoms of acute intoxication are gastrointestinal distress, diarrhea, nausea, and abdominal pain. Vomiting occurs almost universally. The lethal dose in humans is 3–5 g.Decontamination of the gastrointestinal tract after oral uptake of zinc compounds is mostly unnecessary, since patients usually vomit sufficiently. Milk may be administered to decrease absorption of the metal. Zinc levels may be normalized with EDTA salts.
Zinc chloride is extremely detrimental to the lungs, and pulmonary exposure to zinc chloride smoke resulted in ten fatalities.Inhalation of fumes of zinc, zinc oxide, or zinc chloride leads to pulmonary edema and metal fume fever. Onset occurs within 4–6 h and may be delayed up to 8 h. Symptoms include rapid breathing, dyspnea, cough, fever, shivering, sweating, chest and leg pain, myalgias, fatigue, metallic taste, salivation, thirst, and leukocytosis, which can last from 24 to 48 h. In cases of fume inhalation, cortisone preparations should be applied immediately (e.g., by inhalation of Auxiloson) to avoid development of lung edema.
Related articles And Qustion
See also
Lastest Price from Zinc chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7646-85-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $0.00/Kg/Bag2025-04-15
- CAS:
- 7646-85-7
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 10 tons