Properties and Occurrence of Graphite
Graphite is a naturally-occurring form of crystalline carbon. It is a native element mineral found in metamorphic and igneous rocks. Graphite is a mineral of extremes. It is extremely soft, cleaves with very light pressure, and has a very low specific gravity. In contrast, it is extremely resistant to heat and nearly inert in contact with almost any other material. These extreme properties give it a wide range of uses in metallurgy and manufacturing.

Properties
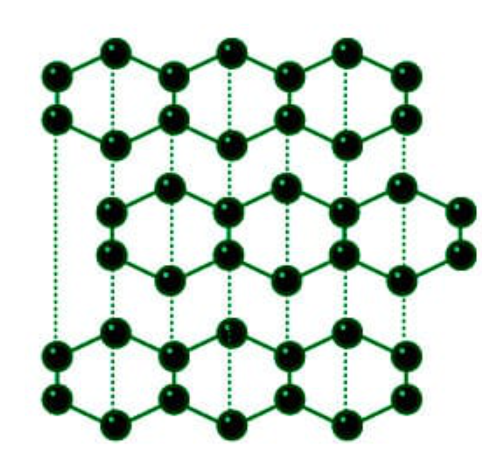
Graphite, an allotropic form of carbon, which exhibits two crystalline structures, hexagonal and rhombohedral, is a highly ordered material (summaries in Bourrat, 2000). It is because of these structures that graphite displays the high degree of anisotropy that determines its properties. Graphite shows a high directional strength and is highly inert, although its reactivity increases as the temperature rises and therefore it reacts with oxygen at temperatures higher than 300–400°C. In most cases it is a polycrystalline material, made up of crystalline aggregates, whose morphology, size, and orientation may vary from one graphite to another.
Occurrence
Graphite is a mineral that forms when carbon is subjected to heat and pressure in Earth's crust and in the upper mantle. Pressures in the range of 75,000 pounds per square inch and temperatures in the range of 750 degrees Celsius are needed to produce graphite. These correspond to the granulite metamorphic facies.
Graphite From Regional Metamorphism (Flake Graphite)
Most of the graphite seen at Earth's surface today was formed at convergent plate boundaries where organic-rich shales and limestones were subjected to the heat and pressure of regional metamorphism. This produces marble, schist, and gneiss that contain tiny crystals and flakes of graphite.
When graphite is in high enough concentrations, these rocks can be mined, crushed to a particle size that liberates the graphite flakes, and processed by specific gravity separation or froth flotation to remove the low-density graphite. The product produced is known as "flake graphite."
Some graphite forms from the metamorphism of coal seams. The organic material in coal is composed mainly of carbon, oxygen, hydrogen, nitrogen, and sulfur. The heat of metamorphism destroys the organic molecules of coal, volatilizing the oxygen, hydrogen, nitrogen, and sulfur. What remains is a nearly pure carbon material that crystallizes into mineral graphite.
This graphite occurs in "seams" that correspond to the original layer of coal. When mined, the material is known as "amorphous graphite." The word "amorphous" is actually incorrect in this usage, as it does have a crystalline structure. From the mine, this material has an appearance similar to lumps of coal without the bright and dull banding.
Synthetic Graphite
"Synthetic graphite" is made by heating high-carbon materials like petroleum coke and coal-tar pitch to temperatures in the range of 2500 to 3000 degrees Celsius. At these high temperatures, all volatile materials and many metals in the feedstock are destroyed or driven off. The graphite that remains links into a sheet-like crystalline structure. Synthetic graphite can have a purity of over 99% carbon, and it is used in manufactured products where an extremely pure material is required.
You may like
Related articles And Qustion
See also
Lastest Price from Graphite manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 7782-42-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton

US $0.00-0.00/KG2025-04-15
- CAS:
- 7782-42-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg