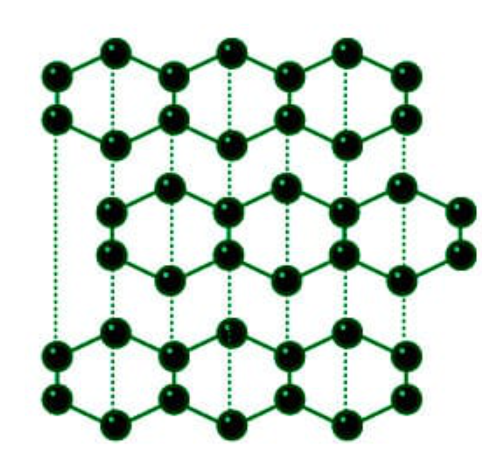
What is graphite metal?
Graphite is a is a form of carbon and a non-metal but the only non-metal can conduct electricity. It is an excellent conductor of heat and electricity and has the highest natural strength and stiffness of any material. It maintains its strength and stability to temperatures in excess of 3,600°C and is very resistant to chemical attack. At the same time it is one of the lightest of all reinforcing agents and has high natural lubricity.

Graphite has various superior properties, for instance, high thermal conductivity, low thermal expansion coefficient, low secondary electron emission coefficient and high emissivity. Thus, it has been researched so far to apply the graphite, for instance, to walls of a plasma container for a nuclear fusion reactor, a collector electrode of an electron tube, and capillaries of an ion laser tube.
As is understood from the fact that the graphite is suitable for walls of a metal fusion furnace and a crucible, the graphite has an inherent property of not reacting with molten metal. However, the graphite reacts on an active metal such as Ti, Zr, Ta and Mo at a temperature over 1,000 degrees centigrade, and thus forms a layer composed of a carbide of these metals at an interface thereof, to thereby firmly bond with the metal.
You may like
Related articles And Qustion
See also
Lastest Price from Graphite manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 7782-42-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton

US $0.00-0.00/KG2025-04-15
- CAS:
- 7782-42-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg