Graphite:Crystal Structure,Properties,Preparation,Uses
Graphite is dark gray to black, opaque, and very soft (with a Mohs scale hardness of 1.5). It is an excellent conductor of heat and electricity. For detailed physical properties of graphite, see the native element (table).
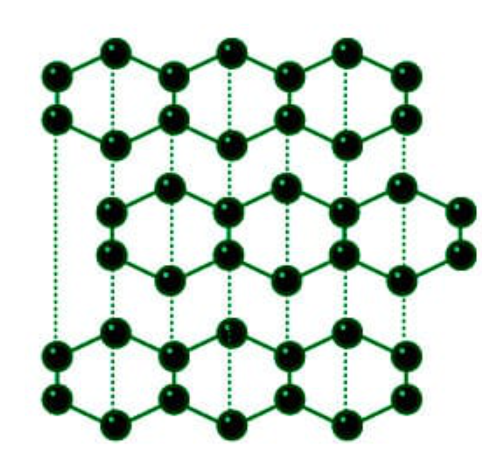
Crystal Structure
Graphite has a layered hexagonal structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets.
Properties
Graphite is a non-metal but has many properties of metals. It is an excellent conductor of heat and electricity and has the highest natural strength and stiffness of any material. It maintains its strength and stability to temperatures over 3,600°C and is very resistant to chemical attack. At the same time, it is one of the lightest of all reinforcing agents and has high natural lubricity.
Preparation
Graphite occurs naturally in igneous and metamorphic rocks, where high temperatures and pressures compress carbon into graphite. Graphite can also be created synthetically by heating materials with high carbon content (e.g. petroleum coke or coal-tar pitch). The carbon-rich material is heated to 2500 to 3000 degrees Celsius, which is hot enough to "purify" the material of contaminants, allowing the carbon to form its hexagonal sheets.
Uses
Natural graphite is mostly used for refractories, batteries, steelmaking, expanded graphite, brake linings, foundry facings, and lubricants.
You may like
Related articles And Qustion
See also
Lastest Price from Graphite manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 7782-42-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton

US $0.00-0.00/KG2025-04-15
- CAS:
- 7782-42-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg




