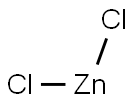Zinc Chloride - Synthesis, Purification and Uses
Zinc Chloride is a chemical compound, which is composed of zinc and chlorine. It is a hygroscopic white crystalline ionic salt with the chemical formula ZnCl2. Zinc Chloride is soluble in mediums such as water, glycerol, ether and alcohol. Since Zinc chloride is a deliquescent, it must be protected from sources of moisture such as water vapor.

Synthesis and Purification
Anhydrous zinc chloride is synthesized by treating zinc with hydrogen chloride.
Zn(s) + 2 HCl → ZnCl2 + H2(g)
Whereas, hydrated and aqueous forms of zinc chloride are prepared by treating zinc with hydrochloric acid. Zinc metal could either be in the form of zinc sulfide or zinc oxide.
ZnS(s) + 2 HCl(aq) → ZnCl2(aq) + H2S(g)
There are impurities present in zinc chloride samples due to hydrolysis. The purification of chloride is simple due to the existence one oxidation state (2+) of zinc. Purification can be done through recrystallization from dioxane (hot). The purification of anhydrous zinc chloride can be done through sublimation with hydrogen chloride gas, followed by the subsequent heating of the sublimate to around 400 °C with dry nitrogen gas. Zinc chloride can also be purified by treating it with thionyl chloride.
Uses
Zinc Chloride has numerous applications in different industries, including pharmaceuticals, health care and paper manufacturing industry. Chemical products are also formulated using zinc chloride.
The uses of zinc chloride, based on the type of the industry are as follows:
1. Chemical industry - Zinc chloride is used in the manufacture of various dyes, intermediate chemicals and solvents such as ethyl acetate.
2. Organic product synthesis - Organic products are synthesized in the laboratory for Lewis acid reaction and various other reactions. It also used as a catalyst in organic processes.
3. Metallurgical Industry - It is used a metal etchant and a metallurgical flux. Zinc chloride is used a flux for the soldering process. It is also used in the manufacture of magnesia cement, which is used as an active ingredient for dental fillings and mouthwashes.
4. Printing and Textile industry - Around 64% zinc chloride in water is used to dissolve silk, cellulose and starch. It finds many other applications such as fire proofing agents and fabric refreshers. Vulcanized fibers are manufactured by soaking paper in concentrated zinc chloride. Zinc chloride is used as a mordant in dyeing and printing materials.
5. Petroleum - Zinc chloride is a powerful emulsion breaker, which separates oil from water.
6. Dry cell - Zinc chloride is used in dry cell batteries as an electrolyte.
7. Other Uses - It is used as a condensing agent, dehydrating agent, wood preservative, deodorant and disinfectant.
Conclusion
Zinc chloride finds numerous applications in various industries, and its scope will increase through research, with the course of time. However, this chemical is known to cause skin irritations, gastrointestinal distress, diarrhea, nausea and pulmonary issues, which can be averted through the adoption of apt safety measures at the chemical manufacturing laboratories and plants.
You may like
Related articles And Qustion
See also
Lastest Price from Zinc chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7646-85-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $0.00/Kg/Bag2025-04-15
- CAS:
- 7646-85-7
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 10 tons