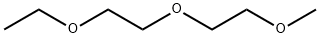Diethylene Glycol Methyl Ethyl Ether: Properties, Synthesis, and Applications
Abstract
Diethylene glycol methyl ethyl ether (DGMEE) has versatile properties. Owing to its strong solvency and stability, it is widely applied across coatings, electronics, printing, agrochemicals, and emerging energy technologies. These characteristics highlight its broad industrial relevance and significant potential for future development.
Introduction
Diethylene glycol methyl ethyl ether is an organic compound with the formula C7H16O3. It is a colorless, practically odorless, poisonous, hygroscopic liquid with a sweetish taste.[1]

Synthesis
Diethylene glycol methyl ethyl ether belongs to the class of polyether alcohols and represents a high-boiling, polar organic solvent within the field of fine organic chemicals. Its synthesis is achieved by using diethylene glycol monoethyl ether (80–100% purity) as the starting material, together with a composite solid alkali formulated at m(NaOH):m(KOH):m(Na₂CO₃):m(K₂CO₃) = 0.5–1.0 : 0–0.5 : 0–0.7 : 0–0.4. Under the conditions of m(solid alkali):m(diethylene glycol monoethyl ether) = 0.5–2.0 : 1.0, with nitrogen flow at 30–120 °C for 1.0–7.0 h, sodium and/or potassium alkoxides are generated. These intermediates subsequently undergo a Williamson ether synthesis at 30–110 °C with halomethanes [m(chloromethane):m(bromomethane):m(iodomethane) = 0–1 : 0–1 : 0–1], applied at m(halomethane):m(diethylene glycol monoethyl ether) = 0.3–1.5 : 1, for 0.5–6.0 h, followed by an aging step of 0.5–5.0 h. Upon completion, the reaction mixture is separated to yield a mother liquor containing diethylene glycol ethyl methyl ether, along with a solid residue enriched in the target compound. The residue is subsequently extracted and washed with methanol at m(methanol):m(residue) = 2.0–10.0 : 0.5–5.0, affording a methanol solution of diethylene glycol ethyl methyl ether. The mother liquor and methanol extract are combined and subjected to fractional distillation. The 50–70 °C fraction allows recovery of methanol, while the 166–180 °C fraction affords the final product, with a purity of 95–99.5% and a water content below 0.1–0.5%.[2]
Application
As an important derivative of ethylene glycol and diethylene glycol, diethylene glycol methyl ethyl ether (DGMEE) has molecular structure that contains both ether linkages and low-carbon alkyl groups (methyl and ethyl), while lacking the strongly polar hydroxyl groups characteristic of ethylene glycol. These structural features endow DEGEE with remarkable solvent properties. It exhibits high solubility not only for small organic molecules such as alcohols, ethers, aldehydes, ketones, aromatics, halogenated hydrocarbons, and alkanes, but also for larger organic compounds and even certain macromolecules, ensuring good solubility and dispersibility. Moreover, DGMEE demonstrates notable solvating ability for selected inorganic compounds, including alkali metal hydroxides and tin oxides. Owing to these properties, DGMEE is widely applied as a high-performance solvent in the synthesis of diverse chemical products, the formulation of advanced cleaning agents, radiation-sensitive resins, conductive coatings, waste polymer recycling, photosensitive printing plates, specialty printing inks, as well as in the surface treatment of mechanical components and printed circuit boards. In the coatings and paints sector, DGMEE serves as a high-performance coalescent in waterborne architectural, industrial, and automotive systems, supporting the global shift toward low-VOC formulations. In electronics, its solvency and stability enable its use in precision cleaning and photoresist stripping during semiconductor and PCB fabrication, particularly in advanced chip production. DGMEE also functions as a solvent in printing inks, enhancing resin compatibility and print quality in packaging applications. In agrochemical formulations, it acts as a carrier solvent that improves the penetration and efficacy of herbicides and fungicides. Moreover, emerging research highlights DGMEE’s promise as a candidate electrolyte solvent in lithium-ion batteries, where its low viscosity and thermal stability can support the performance and safety of next-generation energy storage systems.[3]
Reference
[1] Ataman Chemical. (n.d.). Diethylene glycol methyl ethyl ether. Ataman Chemical. Retrieved from https://www.atamanchemicals.com/diethylene-glycol-methyl-ethyl-ether_u24789/
[2] Yang C.-S. et al. A kind of preparation method of diethylene glycol methyl ethyl ether. CN Patent CN1609085A, published April 27, 2005.
[3] Dataintelo. (n.d.). Global diethylene glycol methyl ethyl ether market report. Dataintelo. Retrieved from https://dataintelo.com/report/global-diethylene-glycol-methyl-ethyl-ether-market
You may like
See also
Lastest Price from Diethylene glycol ethyl methyl ether manufacturers

US $5.00-2.00/KG2025-06-10
- CAS:
- 1002-67-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $0.00/KG2025-05-19
- CAS:
- 1002-67-1
- Min. Order:
- 1000KG
- Purity:
- 99
- Supply Ability:
- 20000