Bis(2-ethylhexyl) adipate: application research and toxicity
Introduction
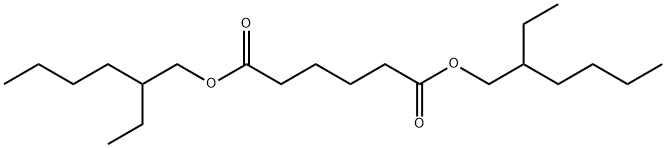
Bis(2-ethylhexyl) adipate (DEHA;Figure.1) is a transparent and colorless oily liquid usually used as a plasticizer in manufacturing of polyvinyl chloride (PVC). Bis(2-ethylhexyl) adipate is also used to generate clear films for food packaging applications. Moreover, it is compatible with ethyl cellulose, nitrocellulose, and most synthetic rubbers. Although it is used widely as a second plasticizer in manufacturing polyvinyl and other polymers, the data about the ecotoxicological and molecular effects of bis(2-ethylhexyl) adipate in the aquatic environment are very limited.Bis(2-ethylhexyl) adipate is one alternative plasticizing chemical to the array of non-phthalate plasticizers, which is an ester of 2-ethylhexanol and adipic acid. The measured solubility of bis(2-ethylhexyl) adipate in water at 20-22℃ ranges from 0.8 to <100 mg/L, which places this substance in the group of the moderately soluble substances. Exposure to neat bis(2-ethylhexyl) adipate did not cause significant irritation or sensitisation reactions in humans . Bis(2-ethylhexyl) adipate shows very little acute toxicity in animal studies. A number of acute studies in algae, crustaceans and fish observed toxicity at concentrations above the solubility of DEHA in water (European Commission Joint Research Centre 2000).However, the acute toxicity for Daphnia magna is shown to be 0.66 mg/L in one study performed with low concentrations, and bis(2-ethylhexyl) adipate is therefore considered very toxic to crustaceans. The chronic data for crustaceans shows that DEHA had adverse effects on the reproduction of D.magna. Bis(2-ethylhexyl) adipate has a measured bioaccumulation factor of 27 showing that DEHA is not a bioaccumulative substance.[1]

Bis(2-ethylhexyl) Adipate Exposure in Zebrafish Larvae
The present study evaluates potential toxic effects of bis(2-ethylhexyl) adipate (DEHA) plasticizer tolarval (72h post fertilization) zebrafish (Danio rerio) by analyzing changes in expression levels of stress-related genes (p53, rad51 and xrcc5) and assessing possible DNA damage of bis(2-ethylhexyl) adipate in larvae. The lethal concentration for 50% mortality (LC50) in larval zebra fish exposed for 96h to 0–200 mg/L bis(2-ethylhexyl) adipate was 89.9±8.03 mg/L. A concentration-dependent increase in DNA strand breaks was detected in cells from larvae exposed for 96h to bis(2-ethylhexyl) adipate.There were some significant differences in induction of stress-related genes in larvae exposed to DEHA relative to control.[1]
Screening Emerging Pollutants in Urine and Nails
Alternative plasticizers and flame retardants (FRs) have been introduced as replacements for banned or restricted chemicals, but much is still unknown about their metabolism and occurrence in humans. Researchers identified the metabolites formed in vitro for four alternative plasticizers (acetyltributyl citrate (ATBC), bis(2-propylheptyl) phthalate (DPHP), bis(2-ethylhexyl) terephthalate (DEHTP), bis(2-ethylhexyl) adipate (DEHA)), and one FR (2,2-bis (chloromethyl)-propane-1,3-diyltetrakis(2-chloroethyl) bisphosphate (V6)). Further, these compounds and their metabolites were investigated by LC/ESI-Orbitrap-MS in urine and finger nails collected from a Norwegian cohort. Primary and secondary ATBC metabolites had detection frequencies (% DF) in finger nails ranging from 46 to 95%. V6 was identified for the first time in finger nails, suggesting that this matrix may also indicate past exposure to FRs as well as alternative plasticizers. Two isomeric forms of DEHTP primary metabolite were highly detected in urine (97% DF) and identified in finger nails, while no DPHP metabolites were detected in vivo. Primary and secondary bis(2-ethylhexyl) adipate metabolites were identified in both matrices, and the relative proportion of the secondary metabolites was higher in urine than in finger nails; the opposite was observed for the primary metabolites. As many of the metabolites present in in vitro extracts were further identified in vivo in urine and finger nail samples, this suggests that in vitro assays can reliably mimic the in vivo processes. Finger nails may be a useful noninvasive matrix for human biomonitoring of specific organic contaminants, but further validation is needed.[2]
The toxicity of bis(2-ethylhexyl) adipate
High levels of bis(2-ethylhexyl) adipate residues were found in house floor dust (1520ng/g) in indoor environments of Guangzhou, China. Bis(2-ethylhexyl) adipate is one of the most widely used plasticizers in food packaging; its residues have been identified in breast milk and food. For example, the analysis of plasticizers in diet samples collected over a span of one week allowed for the estimation of daily intake and revealed an estimated intake concentration of 86 µg/day for Bis(2-ethylhexyl) adipate. Daily plasticizer intake was estimated in Japanese hospital diet samples, and the mean bis(2-ethylhexyl) adipate intakewas 12.5 µg/day. Bis(2-ethylhexyl) adipate concentrations in the marine environment of Kuwait were found to be 0.06-0.13 µg/L in the latest investigation. In Korea, the average concentration of DEHA in industrial bay sediment was 3.48±11.4 ng/g(<iLOQ-71.4). Residues of bis(2-ethylhexyl) adipate in Swedish preschool dust reached 8.5 µg/g in 2018.
There is limited information about DEHA’s health risks or toxicity to people. According to an old report of the Scientific Committee on Emerging and Newly-Identified Health Risks(SCENIHR), Bis(2-ethylhexyl) adipate exposure is likely to cause a reduction in fertility index and cytotoxicity in mice. Bis(2-ethylhexyl) adipate is also slightly irritating to the skin of rabbits. An investigation involving the administration of different doses (0, 200, 400, and 800 mg/kg/day) of DEHA through gavage to pregnant rats reaffirmed the adverse effects on fetal development. Maternal toxicity was observed at the highest dose of 800 mg per kg bw per day. According to an in vitro L929 cell line study, bis(2-ethylhexyl) adipate exhibited nocytotoxicity, whereas its corresponding monoester (MEHA) demonstrated cytotoxic effects at a concentration of 0.05 mg/mL. Furthermore, bis(2-ethylhexyl) adipate led to an extended gestation period at this dosage. Mityata et al. found that exposure to a higher dose of 1000 mg/kg caused disruptions in the estrouscycle and increased ovarian follicle atresia. In general, bis(2-ethylhexyl) adipate is considered a safer alternative to DEHP. There were no antiandrogenic effects or testicular toxicity attributed to DEHA treatment in rats. However, bis(2-ethylhexyl) adipate led to an extended gestation period at a dosage of 800 mg/kg/day, and there was a dose-dependent rise in postnatal mortality at 400 and 800 mg/kg/day. Additionally, bis(2-ethylhexyl) adipate caused a persistent reduction in the body weight of offspring at 800 mg/kg/day.[3]
References
[1]Boran H, Terzi S. Stress-Induced Transcriptional Changes and DNA Damage Associated with Bis(2-ethylhexyl) Adipate Exposure in Zebrafish (Danio rerio) Larvae. Bull Environ Contam Toxicol. 2017;99(3):308-314. doi:10.1007/s00128-017-2116-4
[2]Alves A, Giovanoulis G, Nilsson U, et al. Case Study on Screening Emerging Pollutants in Urine and Nails. Environ Sci Technol. 2017;51(7):4046-4053. doi:10.1021/acs.est.6b05661
[3]Qadeer A, Anis M, Warner GR, et al. Global Environmental and Toxicological Data of Emerging Plasticizers: Current Knowledge, Regrettable Substitution Dilemma, Green Solution and Future Perspectives. Green Chem. 2024;26(10):5635-5683. doi:10.1039/d3gc03428c
You may like
See also
Lastest Price from Bis(2-ethylhexyl) adipate manufacturers

US $230.00/kg2025-05-11
- CAS:
- 103-23-1
- Min. Order:
- 190kg
- Purity:
- 99.5%
- Supply Ability:
- 76 tons

US $0.00/KG2025-04-27
- CAS:
- 103-23-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20tons/month