Synthesis and application of Trimethyl citrate
Introduction
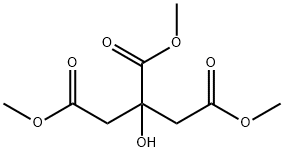
Trimethyl citrate (Figure.1), C9H14O7 (systematic name: trimethyl 2-hydroxypropane-1,2,3-tricarboxylate), was prepared by the esterification of citric acid and methanol in the presence of thionyl chloride at 273 K. Esters of citric acid have received significant attention because of their many applications. Their use as plasticizers has grown because of their low toxicity, compatibility with the host materials and low volatility. They were investigated for use in degradable thermoset polymers. In the biological field, trimethyl citrate is used to synthesize citrate-functionalized ciprofloxacin conjugates and their antimicrobial activities have been determined against a panel of clinically-relevant bacteria. Several different methods and catalysts have been employed for the synthesis of trimethyl citrate from citric acid and methanol using, for example, thionyl chloride and zirconium (IV) dichloride oxide hydrate.[1]

Synthesis of Trimethyl Citrate
Many domestic scholars have reported the synthesis of Trimethyl Citrate as follows.
Catalytic Synthesis of Trimethyl Citrate by Solid Acid
Trimethyl citrate,a kind of non-toxic plasticizer,was prepared by esterification with citric acid and methanol as reactants and solid acid as catalyst. Effects of molar ratio of citric acid to catalyst and molar ratio of citric acid to methanol on the yield were discussed. The results show that the yield of product is up to 91% under the optimum conditions(the fixed reactant time 5 h, the mole ratio of citric acid and methanol 1:4.5~1:5,the mole ratio of citric acid and solid acid 1:0.13).The results of IR analysis and melting point measurement have confirmed that the obtained product is trimethyl citrate.[2]
Catalytic synthesis conditions of trimethyl citrate
The trimethyl citrate was synthesized by citric acid and methanol with sodium hydrogen sulfate as catalyst and toluene as water-carrying agent., The dosage of catalysts, the molar ratio of citric acid and methanol, the reaction temperature and time were explored by single factor experiment and orthogonal experiment. 'The results show that the dosage of catalysts was 1.5g, the molar ratio of citric acid and methanol was 1:6, the reaction temperature was 160℃, and reaction time was 6h, the trimethyl citrate yield was up to 89.6%. The method has advantages of simple operation, lesser environment pollution, higher product purity, and has broad application prospects.[3]
Sodium Bisulfite Catalytic Synthesis of Trimethyl Citrate
Use methanol,citric acid as raw material,under the catalysis of sodium bisulfite to synthesis trimethyl citrate.Through single-factor experiments,investigated the factors influencing on the yield of the product,and finally got the best synthetic process conditions: the reaction time is 5 h,dehydrating agent dosage is 1.1 g,acid glycol ratio is 1:12,catalyst dosage is 4.0 g,stirring speed is 45r/min,and analyzed the influence factors of the low product yield.[4]
Application of trimethyl citrate
A novel low-temperature demulsifier based on trimethyl citrate
A significant amount of water-in-oil (W/O) emulsion is generated during petroleum extraction. However, the current commercial demulsifiers are expensive to produce and requires high demulsification temperatures, leading to increased energy and economic consumption. To enhance the efficiency of demulsifiers and reduce the cost of demulsifying W/O emulsions, researchers have successfully developed a novel demulsifier named TCED through a straightforward two-step process. This demulsifier features trimethyl citrate as the hydrophilic core grafted with three hydrophobic chains. Its structure was characterized using EA, FT-IR and 1H-NMR spectroscopy, and the demulsification performance was comprehensively evaluated. At a low demulsification temperature of 40 °C, TCED demonstrated a remarkable demulsification efficiency (DE) of 99.06% and 98.74% in emulsions containing water contents of 70% (E70) and 50% (E50), respectively. Especially, a DE of 100% could be obtained in both E70 and E50 emulsions at a concentration of 600 mg/L. Moreover, TCED displayed a high DE even at high salinity levels of 50,000 mg/L and across a wide pH range of 2-10. Additionally, the phase interface was consistently clear after demulsification. To investigate the demulsification mechanism of TCED, various adsorption kinetics experiments were conducted, including measurements of interfacial tension (IFT), surface tension (SFT), interfacial competitive adsorption, and stability of interfacial film. The results obtained from the experiments indicated that TCED possessed remarkable diffusion and replacement capabilities within the emulsions. As a result, it effectively disrupted the original interfacial active substances, such as asphaltenes aggregates found in crude oil. TCED exhibits a high DE at low concentration and temperature. This characteristic highlights its significant potential for low-temperature demulsification applications in the petroleum industry.[5]
Acetylacetyl Citrate Trimethyl Ester Synthesis
This study uses diethyl ketone and trimethyl citrate as raw materials to synthesize acetyl acetyl citrate trimethyl ester through ester addition method.It not only uses diethyl ketone as a substance to remove the active hydroxyl group of citrate trimethyl ester,but also generates acetyl acetyl citrate trimethyl ester,a new type of plasticizer.The main research focuses on acetyl citrate trimethyl ester to improve the plasticity,flexibility,and stretchability of polylactic acid.The main research focuses on acetyl citrate trimethyl ester to improve the plasticity, flexibility,and stretchability of polylactic acid.Therefore,the newly obtained acetyl citrate trimethyl ester plasticizer can be added to polylactic acid,and compared with polylactic acid without plasticizers and unmodified citrate trimethyl ester plasticizer. Their various properties can be determined. By analyzing and comparing the properties such as decomposition temperature,glass transition temperature,extraction resistance,and thermal aging, it can be concluded that the new plasticizer has better plasticizing effect,higher resistance to high temperatures,better resistance to extraction,and lower degree of thermal aging.[6]
References
1. Morjan RY, El-Kurdi SM, Azarah JN, et al. Inversion dimers dominate the crystal packing in the structure of trimethyl citrate (trimethyl 2-hy-droxy-propane-1,2,3-tri-carboxyl-ate). Acta Crystallogr E Crystallogr Commun. 2018;74(Pt 9):1362-1365. Published 2018 Aug 24. doi:10.1107/S2056989018011222
2. Huang L,et al.Study on Catalytic Synthesis of Trimethyl Citrate by Solid Acid[J].Liaoning Chemical Industry,2012,41(03):232-233+236.DOI:CNKI:SUN:LNHG.0.2012-03-008.
3. Huang F,et al.Study on catalytic synthesis conditions of trimethyl citrate[J].Journal of Qiqihar University(Natural Science Edition),2019,35(02):64-67.DOI:CNKI:SUN:QQHE.0.2019-02-014.
4. Tian HL,et al.Sodium Bisulfite Catalytic Synthesis of Trimethyl Citrate[J].Shandong Chemical Industry,2023,52(10):54-57+62.DOI:10.19319/j.cnki.issn.1008-021x.2023.10.035.
5. Shen L, Ai G, Liu H, et al. Synthesis and demulsification performance of a novel low-temperature demulsifier based on trimethyl citrate. J Hazard Mater. 2024;472:134543. doi:10.1016/j.jhazmat.2024.134543
6. Zhong L,et al.Study on the Synthesis and Properties of Acetylacetyl Citrate Trimethyl Ester[J].Journal of zhongzhou university,2025,42(02):124-128.DOI:10.13783/j.cnki.cn41-1275/g4.2025.02.019.
You may like
See also
Lastest Price from TRIMETHYL CITRATE manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1587-20-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $100.00-75.00/kg2025-04-21
- CAS:
- 1587-20-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000