Synthesis and application research of 2-Methoxyethyl acrylate
Introduction
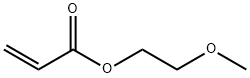
2-Methoxyethyl acrylate is a colorless transparent liquid (Figure 1) with active functional group carbon carbon double bond. It is a highly active functional monomer, which is mainly used to synthesize quaternary ammonium surfactant, polyacrylic acid rubber, coating carrier, textile coating, PVC impact strength improver, pharmaceutical materials, etc. [1]. In particular, 2-Methoxyethyl acrylate is a very useful functional polymer monomer and an important raw material for the synthesis of acrylate rubber. Because the monomer structural unit contains ethylene glycol structure, its homopolymer (PMEA) has become a hydrophilic anticoagulant material. After a large number of experiments and clinical verification, the adsorption amount of blood components such as platelets and plasma proteins on the surface of PMEA shows a low level, showing good blood compatibility. Based on these characteristics, PMEA has been applied to the coating material of small oxygenator (artificial lung) and achieved good clinical results.[2]

Synthesis of 2-Methoxyethyl Acrylate
At present, the synthesis of 2-Methoxyethyl acrylate is mainly catalyzed by protonic acid sulfuric acid, which has long reaction time, many side reactions and serious equipment corrosion, so it is necessary to develop new synthesis methods.
A H3PW12O40 polymer composite film catalyst was prepared, and was characterized by FT-IR. Using it as catalyst, synthesis of 2-Methoxyethyl Acrylate as probe reaction,effects of reactant molar ratio, catalyst dosage, reaction time and catalyst reusedly on catalytic activity were investigated. The catalyst had strong catalytic activity and definite regeneration behavior. The heteropoly acid-polymer composite film was synthesized conveniently by organic-inorganic blending, and can be separated and recovered easily after reaction. Yield of 2-Methoxyethyl Acrylate can reach over 95.4% under optimized conditions.[1]
Other study has provided a practical silicotungstic acid-catalyzed and solvent-free method for the preparation of 2-methoxyethyl acrylate based on the previous research. lt is noteworthy that in this process silicotungstic acid is used as catalyst in homogeneous medium and can be recycled, And also the procedure is easily operated which meets the need of sustainable development.[3]
Application research example
System I
In this study, the MEA/EMA copolymer (P(MEA-EMA)) and MEA/HEMA copolymer (P(MEA-HEMA)) were prepared by solution polymerization with 2-methoxyethyl acrylate(MEA), ethyl methacrylate (EMA) and hydroxyethyl methacrylate (HEMA) as monomers,AIBN as initiator, tetrahydrofuran and dioxane as solvent. The effects of monomer ratio,reaction time, initiator 's concentration and reaction temperature on the properties of the two copolymers were discussed. The polymerization products were characterized by IR, DSC,TG, contact angle measuring instrument, and so on. 2-methoxyethyl acrylate (MEA) and ethyl methacrylate (EMA) were subjected to solution polymerization in System I. With the increasing of the content of MEA gradually,the contact angle of the copolymer decreases and then increases. When the monomer ratio(MEA: EMA) is 4:6, the minimum is 64.44º. The interface free energy decreases and then increases. The Tg decreases and then becomes stable gradually. The molecular weight shows a decreasing trend. The molecular weight distribution gradually broadens, and the processing performance gradually deteriorates. With the increasing of reaction time, the contact angle of the copolymer decreases slightly. When the reaction time is 8h, the minimum is 64.44º. The interfacial free energy decreases gradually. The Tg decreases and then becomes stable gradually broadens, and the molecular weight decreases. The molecular weight distribution changes little. With the increase of initiator concentration, the contact angle of the copolymer decreases and then increases. When the initiator concentration is 1%, the minimum is 61.21°.The interface free energy decreases and then increases. The Tg decreases and then increases,and the molecular weight decreases. The change of molecular weight distribution is small.
System 2
2-methoxyethyl acrylate (MEA) and hydroxyethyl methacrylate (HEMA) were subjected to solution polymerization in System II. With the increasing of HEMA content,the contact angle of the copolymer decreases gradually. When the mixture ratio(MEA:HEMA)is 5:5, the minimum is 43.79°. The interface free energy decreases gradually. The Tg gradually decreases and the molecular weight gradually increases. The molecular weight distribution has no significant change. With the increasing of the reaction time, the contact angle of the copolymer decreases gradually. The decreasing amplitude decreases gradually. When the reaction time is 8h, the minimum is 43.79°. The interfacial free energy decreases gradually. The Tg gradually increases. But the increasing amplitude is small. The change of the molecular weight of the copolymer shows a decreasing trend. And the molecular weight distribution change too much. With the increase of the initiator concentration, the contact angle of the copolymer decreases slightly. When the initiator concentration is 1.5%, the minimum is 36.83°. The interface free energy decreases slightly.The Tg gradually increases. The molecular weight decreases gradually, and the molecular weight distribution changes less. With the increase of the reaction temperature, the contact angle of the copolymer increases gradually. When the reaction temperature is 65°C, the minimum is 32.28°. The interface free energy decreases and then increases, but the amplitude is small. The Tg gradually decreases, and the molecular weight decreases gradually. The molecular weight distribution gradually narrowed.[2]
References
[1] Xiong Qi,et al.Synthesis of 2-Methoxyethyl Acrylate Catalyzed by Heteropolyacid-polymer Composite Fim[J].Guangdong Chemical Industry,2012,39(05):313-314,317.
[2] Zhou Wei. Study on the synthesis and properties of 2-methoxyethyl acrylate copolymer[D].Jiangsu University of Science and Technology,2017.
[3] Xu Yu,et al.Green Synthesis of 2-Methoxyethyl Acrylate[J].Journal of Hangzhou Normal University(Natural Science Edition) ,2010,9(05):361-362,391.
You may like
See also
Lastest Price from 2-Methoxyethyl acrylate manufacturers

US $0.00/kg2025-04-15
- CAS:
- 3121-61-7
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 10 toms
US $1.00/KG2024-08-11
- CAS:
- 3121-61-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T