Lapatinib: Uses and applications, ADME profiles and Side effect
Introduction
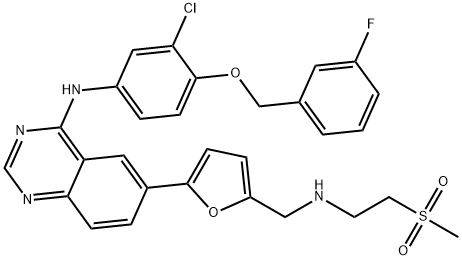
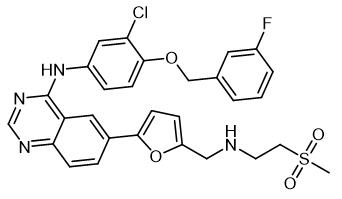
Lapatinib,systematic chemical names is N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino) methyl]furan-2-yl]quinazolin-4-amine (Empirical Formula: C29H26ClFN4O4S).Molecular Weight is 581.1 g/mol. Lapatinib is pale yellow to yellow powder(Figure.1).pKa1=3.80, pKa2=7.20; Lapatinib is very poorly soluble in water (0.007mg/mL) and in 0.1N HCl is 0.001mg/mL; Soluble in DMSO at 200mg/mL [1].

Uses and applications
Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors, which dually inhibits the growth factor receptors ErbB1 (epidermal growth factor receptor, EGFR) and ErbB2 (HER2). It is approved by the FDA in 2007 for the use in patients with advanced metastatic breast cancer in conjunction with the chemotherapy drug capecitabine or with letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer.The recommended dosage for advanced or metastatic breast cancer is1250mg (5 tablets), orally once daily for 21 days in combination with capecitabine 2000mg/m2/day (administered orally in 2 doses approximately 12 h apart) on Days 1-14 in a repeating 21-day cycle. The recommended dose for hormone receptor-positive, HER2-positive metastatic breast cancer is 1500mg (6 tablets) given orally once daily continuously in combination with letrozole 2.5mg once daily. The lapatinib should be taken at least 1 h before or 1 h after a meal.
Absorption
Absorption following oral administration of lapatinib is incomplete andvariable. Peak plasma concentrations (Cmax) of lapatinib are achieved approximately 3 h after administration. Steady state reached within 6–7 days following daily dosing of lapatinib. The systemic exposure to lapatinib was increased with food, particularly with high-fat meal.[1]
Distribution
Lapatinib is highly bound (greater than 99%) to albumin and alpha-1 acid glycoprotein. In vitro studies have indicate that lapatinib is a substrate for and inhibitor of the transporters breast cancer-resistance protein (BCRP,ABCG2) and P-glycoprotein (P-gp, ABCB1). The volume of distribution (Vd/L) of the terminal phase of lapatinib was >2200 L.
Metabolism
Lapatinib undergoes extensive metabolism, primarily by the cytochromeP450 (CYP) 3A4, 3A5, 2C19, and 2C8 isozymes.
Elimination
Lapatinib is eliminated predominantly through metabolism by CYP3A4/5 with negligible renal excretion. At clinical doses, the terminal phase half-life following a single dose was 14.2 h. Recovery of parent lapatinib in feces ranging from 3% to 67% of an oral dose.[1]
Side-Effect
Lapatinib has been administered to almost 9,000 patients in clinical studies. The most frequently reported adverse events are gastrointestinal disorders (e.g., diarrhea, nausea, vomiting) and dermatologic disorders (e.g., rash, hand-foot syndrome, dry skin) . The adverse events generally are not life threatening; however, failure to adequately manage the events may diminish quality of life, decrease treatment adherence, and potentially result in treatment discontinuation.Proactive education, intense surveillance, and early intervention by oncology nurses in the management of diarrhea are required to maintain quality of life and treatment adherence.
Diarrhea and dermatologic adverse events are reported commonly by patients treated with lapatinib. Diarrhea can range from mild to severe based on the agents used in combination with lapatinib. The adverse events may diminish quality of life, reduce treatment adherence, and lead to discontinuation of therapy. Consequently, proactive management of diarrhea is crucial, especially inpatients receiving lapatinib in combination with other agents that also cause diarrhea. As the utility of lapatinib expands,crucial proactive diarrhea-management and dose-reduction strategies are evolving to decrease the likelihood of grade 3 or4 toxicity. With regard to dermatologic adverse events, most are mild to moderate in severity, are of limited duration, and frequently do not require treatment intervention. However, in some patients, management of dermatologic adverse events is of great importance. This article reviews data regarding diarrhea and dermatologic adverse events in patients treated with lapatinib and summarizes the key role that oncology nurses play in educating patients about the potential for adverse events and the importance of preventive measures, ongoing surveillance, appropriate treatment, and dose reductions.[2]
References
[1]Abdelgalil AA, Alkahtani HM. Lapatinib: A comprehensive profile. Profiles Drug Subst Excip Relat Methodol. 2023;48:135-166. doi:10.1016/bs.podrm.2022.11.005
[2[Frankel C, Palmieri FM. Lapatinib side-effect management. Clin J Oncol Nurs. 2010;14(2):223-233. doi:10.1188/10.CJON.223-233
You may like
Related articles And Qustion
Lastest Price from Lapatinib manufacturers

US $0.00-0.00/mg2025-06-28
- CAS:
- 231277-92-2
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000

US $5.00-0.50/KG2025-06-05
- CAS:
- 231277-92-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS